
EMG1 interacts with NOP14 to regulate the growth, migration, and invasion of melanoma cells via the Wnt/β-catenin pathway
Introduction
Malignant melanoma, which originates from melanocytes located in the skin or mucous membranes, is an invasive cancer with a high fatality rate (1). The incidence of malignant melanoma has increased steadily in recent years, giving it one of the highest mortality rates among human cancers (1,2). Age and family history, exposure to ultraviolet radiation, fair skin, and dysplastic nevi syndrome are the known risk factors for malignant melanoma progression (3), and the typical characteristics of melanoma are early metastasis and uncontrolled growth (4). Surgery, radiation therapy, and chemotherapy, the current therapeutic interventions for metastatic melanoma, are insufficient to achieve durable responses (5). Therefore, combinations of treatment modalities, including immunotherapies and molecularly targeted therapies, must be explored for the treatment of advanced melanoma (6,7). Although some treatments have shown clinical benefit, understanding the mechanism of melanoma progression will reveal additional targets (8). Hence, identifying new biomarkers and exploring the mechanisms involved in melanoma progression are critical for the development of novel melanoma therapies.
Nucleolar complex protein 14 (NOP14) is a nucleolar stress response protein responsible for 18S rRNA maturation and 40S ribosome production (9). Because ribosomes play an important role in DNA repair and in the regulation of cell proliferation, disorders of ribosome function can lead to tumor formation (10). Mounting evidence suggests that NOP14 is involved in the development of cancer. For instance, overexpression of NOP14 promoted the proliferation and metastasis of pancreatic cancer lines in vitro and in vivo (11). By inhibiting the nuclear receptor-interacting protein 1 (NRIP1)/Wnt/β-catenin pathway, NOP14 suppressed oncogenesis and metastasis in a breast cancer model (12). Warda et al. identified a nucleolar subcomplex containing the pre-ribosomal factors NOP14, nucleolar complex protein 4 homolog (NOC4L), UTP14A, and EMG1, which was required for its recruitment to nucleoli. The D86G mutation of EMG1, which is associated with Bowen-Conradi syndrome, resulted in its decreased nucleolar localization, proteasome-dependent degradation, and accumulation in nuclear foci. In addition to its role in nuclear import, binding of the importin (Imp) β/7 heterodimer to EMG1 can prevent its non-specific aggregation with RNAs in vitro, suggesting that importins may act as chaperones by binding to RNA methyltransferases (13). Furthermore, EMG1 is involved in ribosome biogenesis. The striking phenotype in lymphoblasts compared with fibroblasts suggests a greater need for EMG1 in rapidly dividing cells (14). However, the role of NOP14 and EMG1 in melanoma development remains largely exclusive.
The Wnt/β-catenin axis orchestrates numerous biological processes, including cell proliferation, differentiation, tissue regeneration, organogenesis, and tumorigenesis (15-19). Targeting the versatile Wnt/β-catenin pathway is considered a promising potential strategy for cancer therapy (20,21). β-catenin is a key transducer of Wnt signaling (22,23). A complex consisting of adenomatous polyposis coli (APC), casein kinase 1α (CK1α), glycogen synthase kinase 3α/β (GSK-3α/β), and AXIN1 tightly controls β-catenin by phosphorylation-mediated proteolysis (22,24-27). Some studies have suggested that during melanomagenesis, the β-catenin transcription factor is frequently activated via non-mutational alterations such as reduced expression of CK1α, leading to β-catenin protein stabilization (28,29). Some studies indicated that β-catenin inhibits melanoma cell invasiveness, and the loss of β-catenin predicts poor survival in melanoma patients (30-33). Other studies showed both in vitro and in vivo that increased abundance or stabilization of β-catenin results in increased melanoma metastasis (34-38).
In this study, we found that EMG1 interacts with NOP14 to regulate the growth, migration, and invasion of melanoma cells by regulating Wnt/β-catenin signaling. These findings may suggest EMG1 and NOP14 as novel targets for the treatment of melanoma.
Methods
Cell culture and treatment
The human melanoma cell lines A375 and SK-MEL-1 were from the Cell Bank of the Chinese Academy of Sciences (Shanghai). As previously reported (39), all cell lines were cultured in DMEM (Thermo Fisher Scientific, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 100 U/mL each of penicillin and streptomycin (Gibco/Thermo Fisher Scientific, USA) at 37 °C in a humidified atmosphere with 5% CO2. Full-length human cDNAs were amplified by PCR to construct EMG1- or NOP14-encoding plasmids using the pcDNA3.1 vector as the backbone (Realgene, China). Cells (1×105 cells/well) were plated in 24-well plates and transfected with NOP14 or EMG1 overexpression plasmids using FuGENE HD transfection reagent (Roche Applied Science, USA). The cells were harvested for analysis after 48 h of transfection.
Migration and invasion assays
Cells (2×105) were plated in the upper wells of Transwell inserts (Corning, USA) with (for invasion experiments) or without (for migration experiments) 10 mg/mL Matrigel (BD Biosciences, USA). The lower wells of the Transwell chambers were filled with culture medium containing 10% FBS. Non-migrating cells on the upper side of the filter were removed by wiping with a cotton swab after 48 h of incubation at 37 °C in a humidified atmosphere containing 5% CO2. The membranes were fixed with 70% cold ethanol, and cells were stained with 0.1% crystal violet. Cell counting was performed under 200× magnification using a light microscope (Olympus, Japan). The experiment was repeated three times.
Western blotting
Cells were lysed in ice-cold mammalian radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China) containing a protease inhibitor cocktail (Invitrogen/Thermo Fisher Scientific, USA). Protein quantification was performed using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific). SDS-PAGE was used to separate equal amounts of protein, which were transferred onto polyvinylidene fluoride (PVDF) membranes (Thermo Fisher Scientific) and then incubated with 10% non-fat milk overnight at 4 °C. Membranes were washed three times with phosphate-buffered saline (PBS) containing Tween 20 (PBST) and then incubated for 1 h at room temperature with the following primary antibodies: EMG1 (1:1,000), NOP14 (1:500), WNT3a (1:800), β-catenin (1:1,000), GSK-3β (1:500), and GAPDH (1:2,000). The antibodies were all purchased from Abcam (USA). After washing three times with PBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG H + L secondary antibodies (Southern Biotech, USA) at a 1:5,000 dilution. An enhanced chemiluminescence (ECL) detection kit (Thermo Fisher Scientific) was used to visualize protein bands.
Immunoprecipitation
Protein from 1×107 A375 or SK-MEL-1 cells was extracted in 1 mL RIPA buffer containing 0.5% NP-40, 1.0% Triton X-100, and protease inhibitors, and was collected by centrifugation at 14,000 ×g for 15 min at 4 °C. The extract was precleared by incubation with 5 µg of normal goat IgG. The interacting proteins were co-immunoprecipitated (CoIPed) using EMG1 and NOP14 antibodies (Abcam), and SDS-PAGE was performed for western blotting.
GST pulldown assays
NOP14 and EMG1 expression plasmids were cloned into the pGEX-2T prokaryotic expression vector downstream of the glutathione S-transferase (GST) sequence. Then, plasmids were used to transform the E. coli BL21 strain, and protein expression was induced using 1 mM isopropylthiogalactoside (IPTG) for 1 h at 37 °C. Bacteria were then pelleted and resuspended in PBS supplemented with complete protease inhibitors (Roche Applied Science), and lysates were collected by centrifugation (10,000 ×g, 15 min, 4 °C). Purified GST-NOP14 and -EMG1 proteins were incubated with beads coupled with approximately 5 µg glutathione-sepharose. Proteins bound to the beads were separated by 10% SDS-PAGE, and gels were stained with Coomassie brilliant blue R-250. Bands representing putative NOP14- and EMG1-binding proteins were recovered, digested by trypsinization, and analyzed by western blotting.
Flow cytometry
Apoptosis was assessed using an annexin V and propidium iodide (PI) dual staining kit (Biolegend, USA) according to the manufacturer’s instructions. Briefly, cells (5×103) were resuspended in 500 mL binding buffer. Then, 5 µL each of annexin V-FITC and PI was added to the cell suspension. After 15 min of incubation at room temperature, the cells were analyzed by flow cytometry. The experiment was repeated in triplicate.
Statistical analysis
SPSS 21.0 software (IBM, USA) was used for all statistical analysis. Data represent the mean ± standard deviation (SD). Student’s t-tests were used for comparisons of two groups, and one-way analysis of variance (ANOVA) was used for multiple group comparisons. The correlations between EMG1 protein levels and clinicopathological characteristics of melanoma patients were analyzed by chi-squared tests. P<0.05 was considered statistically significant.
Results
EMG1 expression is associated with melanoma
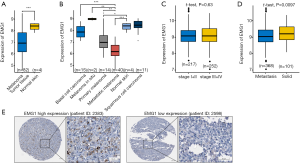
EMG1 expression in the Riker melanoma dataset was analyzed using the public database Oncomine (40). EMG1 mRNA expression was significantly lower in melanoma than in normal skin (Figure 1A). Further, we downloaded the cohort (GSE7553) and analyzed the subtype groups. The results showed that EMG1 was downregulated in primary melanoma comparing to normal skin, and the level of EMG1 in metastatic melanoma was further decreased, comparing to other melanoma types (Figure 1B). Moreover, we explored TCGA database to analysis the expression levels of EMG1 and found that the expression level of EMG1 showed no significant difference between the groups of stage I + II and III + IV patients (Figure 1C). However, the expression level was significantly downregulated in patient group with metastasis (Figure 1D). In the Human Protein Atlas (HPA) database (41), a patient (ID: 2598) with melanoma progression had low EMG1 levels, while a non-progressing patient (ID: 2383) had high EMG1 progression (Figure 1E). Using online tools from PROGgene (42), oncoLnc (43), HPA and Gepia (44), the overall survival rate was analyzed between patients with different expression levels of EMG1, however, the results all showed no significance (data not shown). Taken together, these mining data reveal that EMG1 is downregulated during melanoma development.
EMG1 overexpression inhibits melanoma cell proliferation, migration, and invasion
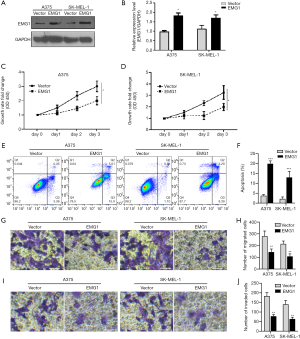
To evaluate the functional role of EMG1 in melanoma, EMG1 was overexpressed in two melanoma cell lines, A375 and SK-MEL-1. The upregulation of EMG1 was confirmed by western blotting (Figure 2A,B). Overexpression of EMG1 significantly inhibited the growth of the two melanoma cell lines (Figure 2C,D). Overexpression of EMG1 also significantly increased apoptosis (Figure 2E,F), while migration (Figure 2G,H) and invasion (Figure 2I,J) were both inhibited by EMG1 overexpression. Taken together, these data indicate that EMG1 inhibits proliferation, migration, and invasion in melanoma cells.
EMG1 associates with NOP14
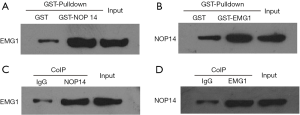
A potential interaction between EMG1 and NOP14 has been reported; however, this interaction has not been evaluated in melanoma. EMG1-interacting proteins were therefore evaluated by GST pulldown and CoIP assays in melanoma cells. For GST pulldown assays, two GST fusion plasmids, GST-EMG1 and GST-NOP14, were constructed. EMG1 was pulled down with GST-NOP14 (Figure 3A), and NOP14 was pulled down with GST-EMG1 (Figure 3B). Furthermore, EMG1 CoIPed with an NOP14 antibody (Figure 3C), and NOP14 CoIPed with an EMG1 antibody (Figure 3D). Taken together, these data suggest that EMG1 interacts with NOP14 in melanoma cells.
EMG1 and NOP14 function together to suppress the progression of melanoma
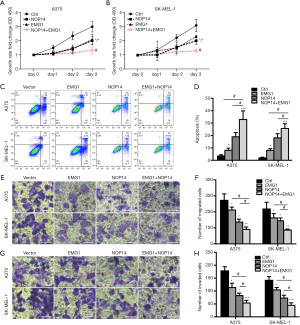
After determining that EMG1 interacts with NOP14 in melanoma cells, we asked whether EMG1 and NOP14 act together to control the progression of melanoma. A375 and SK-MEL-1 melanoma cells overexpressing EMG1, NOP14, or the combination were generated using previously verified overexpression plasmids (39). Cell proliferation, apoptosis, migration, and invasion were observed. As shown in Figure 4A and B, compared with the control group, A375 cells overexpressing NOP14 or EMG1 showed significantly decreased proliferation, while cells overexpressing both NOP14 and EMG1 showed a further decrease in proliferation (Figure 4A). Similar results were obtained with the SK-MEL-1 cells (Figure 4B). The apoptosis rate in both cell lines was also significantly increased by overexpression of EMG1 or NOP14, and the strongest effects were observed when both proteins were overexpressed together (Figure 4C,D). Similar trends were observed with respect to cell migration (Figure 4E,F) and invasion (Figure 4G,H). These data suggest that EMG1 and NOP14 act together to inhibit cell proliferation, migration, and invasion in melanoma cells.
EMG1 and NOP14 modulate Wnt/β-catenin signaling in melanoma cells
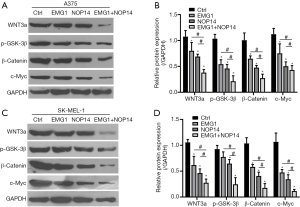
After validating the functional role of EMG1 and NOP14 in melanoma, we evaluated the detailed mechanism through which EMG1 and NOP14 alter melanoma development. It has been reported that the Wnt/β-catenin signaling pathway plays an important role in cancer progression, including in melanoma. In our previous studies, we evaluated changes in the mRNA and protein levels of genes encoding various components of the Wnt/β-catenin pathway in response to overexpression of NOP14 in melanoma cell lines (39). We asked whether EMG1 and NOP14 would show increased effects on this same pathway. The levels of WNT3a, β-catenin, and phosphorylated GSK3β (p-GSK-3) were evaluated by western blotting. In addition, the level of c-Myc, a transcriptional target of the Wnt/β-catenin pathway that is thought to modulate the expression of genes involved in melanoma development, was also evaluated (45). As shown in Figure 5, overexpression of NOP14, alone or together with EMG1, in the A375 cell line significantly suppressed the expression of WNT3a, p-GSK-3β, β-catenin, and c-Myc. In addition, the levels of these proteins decreased more significantly in cells in which both NOP14 and EMG1 were overexpressed (Figure 5A,B). Similar results were observed in the SK-MEL-1 cell line (Figure 5C,D). These results suggest that EMG1 and NOP14 act together to regulate melanoma development by modulating the Wnt/β-catenin signaling pathway.
Conclusions
In recent years, there has been accumulating evidence that NOP14 is involved in tumor progression, cell proliferation, migration, invasion, and apoptosis (46-49). In a previous study, we found that in malignant melanoma, NOP14 was downregulated compared with the level of expression in melanocytes (50). In addition, we identified a significant correlation between NOP14 expression and melanoma thickness and lymph node metastasis. NOP14 is a nuclear protein involved in the processing of pre-18S rRNA and in small ribosomal subunit assembly (9). Its function is highly conserved in eukaryotic organisms (51). NOP14 is essential for 40 s ribosome maturation. Heat stress can cause a sharp decrease in the expression of NOP14 mRNA, thereby reducing the level of 40 s ribosomal subunits (51). Nop14p, the yeast homolog of human NOP14 (9,52), is a small subunit (SSU) biogenesis factor that is a component of the pre-90S particle (53). Loss of Nop14p decreased the levels of 20S and 27SA2 pre-rRNA, and increased the production of 35S and 23S pre-rRNA (9,52). NOP14 is known to regulate the expression of EMG1 [also known as Nep1 (54)]. EMG1 is involved in processing rRNA precursor molecules into 18s RNA, and EMG1 interacts with NOP14 to promote the maturation of 18sRNA and the production of 40sRNA (51). The yeast ortholog of EMG1 is required for the biogenesis of small ribosomal subunits, which are involved in the hyper-modification of uridine 1191 of 18S rRNA (51,55,56). Consistent with this, EMG1 was recently found to bind to this site in the 90S pre-ribosomes of Chaetomium thermophilum by cryo-electron microscopy (cryo-EM) (57,58).
After further evaluation of the functions of EMG1 in the A375 and SK-MEL-1 melanoma cell lines, we hypothesized that EMG1 overexpression suppressed melanoma cell proliferation. EMG1 overexpression also increased apoptosis in both melanoma cell lines. In Transwell assays, EMG1 overexpression decreased the migratory capacity and invasiveness of A375 and SK-MEL-1 cells. These data suggest that EMG1 plays an important role in melanoma formation and development.
The Wnt proteins are widely expressed in invertebrates and vertebrates, and are highly conserved during species evolution. Wnt signaling plays a crucial role in the early development of animal embryos, organ formation, tissue regeneration, and other physiological processes (59). It is now widely accepted that inappropriate positioning of β-catenin in the nucleus is a key carcinogenic process. β-catenin is susceptible to transfer to the nucleus, and systems that regulate nuclear β-catenin entry are multifactorial and highly dependent on the environment (60). Recent studies have determined that melanoma cells develop an efficient new mechanism to activate the β-catenin signaling pathway by suppression of CK1α expression, defining CK1α as a novel tumor suppressor in melanoma (28,61). The activity of β-catenin is mainly determined by regulation of its proteolysis by the β-catenin destruction complex, which includes CK1α, GSK-3, APC, and AXIN1 (26,62). Constitutive activation of signal transducer and activator of transcription 3 (STAT3) promotes survival in a wide spectrum of human cancers, including malignant melanoma (63). The study of these mechanisms may have important therapeutic implications. The activity of the Wnt/β-catenin signaling pathway depends primarily on the activity of GSK-3, which controls β-catenin stability/degradation (64). GSK-3-dependent phosphorylation of β-catenin restricts its nuclear translocation by inducing proteasome-dependent proteolysis (65). In addition, it has been reported that the Wnt/β-catenin signaling pathway also contributes to metastasis in melanoma (66). Here, we show that co-expression of EMG1 and NOP14 inhibits the Wnt/β-catenin pathway to suppress melanoma development. Targeting EMG1 and NOP14 may therefore be a potential strategy for the treatment of melanoma patients with abnormally activated Wnt/β-catenin signaling.
In conclusion, we report that EMG1 is downregulated in malignant melanoma. EMG1 and NOP14 overexpression suppresses melanoma cell proliferation, promotes apoptosis, and inhibits cell migration and invasion. Additionally, overexpression of EMG1 and NOP14 decreases the level of WNT3a, p-GSK-3β, β-catenin, and c-Myc. Thus, EMG1 interacts with NOP14 to reduce cell proliferation and metastasis by regulating the Wnt/β-catenin signaling pathway in melanoma. These data may provide new support for the development of therapeutic agents targeting these molecules in melanoma.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.79). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, and will ensure that all questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cohen-Solal KA, Kaufman HL, Lasfar A. Transcription Factors as Critical Players in Melanoma Invasiveness, Drug Resistance and Opportunities for Therapeutic Drug Development. Pigment Cell Melanoma Res 2018;31:241. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953-2008--are recent generations at higher or lower risk? Int J Cancer 2013;132:385-400. [Crossref] [PubMed]
- Tas F, Erturk K. Recurrence behavior in early-stage cutaneous melanoma. Melanoma Res 2017;27:134. [Crossref] [PubMed]
- Berrocal A, Cabañas L, Espinosa E, et al. Melanoma: Diagnosis, Staging, and Treatment. Consensus group recommendations. Adv Ther 2014;31:945-60. [Crossref] [PubMed]
- George BPA, Abrahamse H, Hemmaragala NM. Caspase dependent apoptotic inhibition of melanoma and lung cancer cells by tropical Rubus extracts. Biomed Pharmacother 2016;80:193-9. [Crossref] [PubMed]
- Lancet T. Melanoma research gathers momentum. Lancet 2015;385:2323. [Crossref] [PubMed]
- Singh S, Zafar A, Khan S, et al. Towards therapeutic advances in melanoma management: An overview. Life Sci 2017;174:50-8. [Crossref] [PubMed]
- Liu PCC, Thiele DJ. Novel Stress-responsive Genes EMG1 and NOP14 Encode Conserved, Interacting Proteins Required for 40S Ribosome Biogenesis. Mol Biol Cell 2001;12:3644-57. [Crossref] [PubMed]
- Ruggero D, Grisendi S, Piazza F, et al. Dyskeratosis Congenita and Cancer in Mice Deficient in Ribosomal RNA Modification. Science 2003;299:259. [Crossref] [PubMed]
- Zhou B, Wu Q, Chen G, et al. NOP14 promotes proliferation and metastasis of pancreatic cancer cells. Cancer Lett 2012;322:195-203. [Crossref] [PubMed]
- Lei JJ, Peng RJ, Kuang BH, et al. NOP14 suppresses breast cancer progression by inhibiting NRIP1/Wnt/β-catenin pathway. Oncotarget 2015;6:25701-14. [Crossref] [PubMed]
- Warda AS, Freytag B, Haag S, et al. Effects of the Bowen-Conradi syndrome mutation in EMG1 on its nuclear import, stability and nucleolar recruitment. Hum Mol Genet 2016;25:5353-64. [PubMed]
- Armistead J, Hemming R, Patel N, et al. Mutation of EMG1 causing Bowen-Conradi syndrome results in reduced cell proliferation rates concomitant with G2/M arrest and 18S rRNA processing delay. Bba Clin 2014;1:33-43. [Crossref] [PubMed]
- Acebron SP, Karaulanov E, Berger BS, et al. Mitotic Wnt Signaling Promotes Protein Stabilization and Regulates Cell Size. Mol Cell 2014;54:663-74. [Crossref] [PubMed]
- Atlasi Y, Noori R, Gaspar C, et al. Wnt Signaling Regulates the Lineage Differentiation Potential of Mouse Embryonic Stem Cells through Tcf3 Down-Regulation. PLoS Genet 2013;9:e1003424. [Crossref] [PubMed]
- Clevers H, Loh KM, Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014;346:1248012. [Crossref] [PubMed]
- Green JL, Inoue T, Sternberg PW. Opposing Wnt Pathways Orient Cell Polarity during Organogenesis. Cell 2008;134:646-56. [Crossref] [PubMed]
- Polakis P. Wnt Signaling in Cancer. Cold Spring Harb Perspect Biol 2012;4:a008052. [Crossref] [PubMed]
- Dzobo K, Thomford NE, Senthebane DA. Targeting the Versatile Wnt/beta-Catenin Pathway in Cancer Biology and Therapeutics: From Concept to Actionable Strategy. OMICS 2019;23:517-38. [Crossref] [PubMed]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science 2000;287:1606-9. [Crossref] [PubMed]
- Ashford S, Williard J. Osteoarthritis: A review. Nurse Pract 2014;39:1-8. [Crossref] [PubMed]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009;17:9-26. [Crossref] [PubMed]
- Gao ZH, Seeling JM, Hill V, et al. Casein kinase I phosphorylates and destabilizes the ?-catenin degradation complex. Proc Natl Acad Sci U S A 2002;99:1182-7. [Crossref] [PubMed]
- Ha NC, Tonozuka T, Stamos JL, et al. Mechanism of Phosphorylation-Dependent Binding of APC to β-Catenin and Its Role in β-Catenin Degradation. Mol Cell 2004;15:511-21. [Crossref] [PubMed]
- Liu C, Li Y, Semenov M, et al. Control of beta-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002;108:837-47. [Crossref] [PubMed]
- Stamos JL, Weis WI. The beta-Catenin Destruction Complex. Cold Spring Harb Perspect Biol 2013;5:a007898. [Crossref] [PubMed]
- Sinnberg T, Menzel M, Kaesler S, et al. Suppression of casein kinase 1alpha in melanoma cells induces a switch in beta-catenin signaling to promote metastasis. Cancer Res 2010;70:6999-7009. [Crossref] [PubMed]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017;36:1461-73. [Crossref] [PubMed]
- Arozarena I, Bischof H, Gilby D, et al. In melanoma, beta-catenin is a suppressor of invasion. Oncogene 2011;30:4531-43. [Crossref] [PubMed]
- Chien AJ, Moore EC, Lonsdorf AS, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A 2009;106:1193-8. [Crossref] [PubMed]
- Kageshita T, Hamby CV, Ishihara T, et al. Loss of beta-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol 2001;145:210-6. [Crossref] [PubMed]
- Maelandsmo GM, Holm R, Nesland JM, et al. Reduced beta-catenin expression in the cytoplasm of advanced-stage superficial spreading malignant melanoma. Clin Cancer Res 2003;9:3383-8. [PubMed]
- Damsky WE, Curley DP, Santhanakrishnan M, et al. beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 2011;20:741-54. [Crossref] [PubMed]
- Murakami T, Toda S, Fujimoto M, et al. Constitutive activation of Wnt/beta-catenin signaling pathway in migration-active melanoma cells: role of LEF-1 in melanoma with increased metastatic potential. Biochem Biophys Res Commun 2001;288:8-15. [Crossref] [PubMed]
- Zuidervaart W, Pavey S, van Nieuwpoort FA, et al. Expression of Wnt5a and its downstream effector beta-catenin in uveal melanoma. Melanoma Res 2007;17:380-6. [Crossref] [PubMed]
- Grossmann AH, Yoo JH, Clancy J, et al. The small GTPase ARF6 stimulates beta-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci Signal 2013;6:ra14. [Crossref] [PubMed]
- Vaid M, Prasad R, Sun Q, et al. Silymarin targets beta-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS One 2011;6:e23000. [Crossref] [PubMed]
- Li J, Fang R, Wang J, et al. NOP14 inhibits melanoma proliferation and metastasis by regulating Wnt/beta-catenin signaling pathway. Braz J Med Biol Res 2018;52:e7952. [Crossref] [PubMed]
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004;6:1-6. [Crossref] [PubMed]
- Pontén F, Jirström K, Uhlen M. The Human Protein Atlas--a tool for pathology. J Pathol 2008;216:387-93. [Crossref] [PubMed]
- Goswami CP, Harikrishna N. PROGgeneV2: enhancements on the existing database. BMC Cancer 2014;14:970. [Crossref] [PubMed]
- J A. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Science 2016;2:e67. [Crossref]
- Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98-W102. [Crossref] [PubMed]
- Postel EH, Berberich SJ, Flint SJ, et al. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science 1993;261:478-80. [Crossref] [PubMed]
- Kühn H, Hierlmeier T, Merl J, et al. The Noc-domain containing C-terminus of Noc4p mediates both formation of the Noc4p-Nop14p submodule and its incorporation into the SSU processome. PLoS One 2009;4:e8370. [Crossref] [PubMed]
- Zhou B, Irwanto A, Guo YM, et al. Exome sequencing and digital PCR analyses reveal novel mutated genes related to the metastasis of pancreatic ductal adenocarcinoma. Cancer Biol Ther 2012;13:871-9. [Crossref] [PubMed]
- Cao Q, Wang X, Zhao M, et al. The Central Role of EED in the Orchestration of Polycomb Group Complexes. Nat Commun 2014;5:3127. [Crossref] [PubMed]
- Woods NT, Mesquita RD, Michael S, et al. Charting the landscape of tandem BRCT domain-mediated protein interactions. Sci Signal 2012;5:rs6. [Crossref] [PubMed]
- Yu Y, Gao F, He Q, et al. lncRNA UCA1 Functions as a ceRNA to Promote Prostate Cancer Progression via Sponging miR143. Mol Ther Nucleic Acids 2020;19:751-8. [Crossref] [PubMed]
- Liu PC, Thiele DJ. Novel stress-responsive genes EMG1 and NOP14 encode conserved, interacting proteins required for 40S ribosome biogenesis. Mol Biol Cell 2001;12:3644-57. [Crossref] [PubMed]
- Milkereit P, Strauss D, Bassler J, et al. A Noc complex specifically involved in the formation and nuclear export of ribosomal 40 S subunits. J Biol Chem 2003;278:4072-81. [Crossref] [PubMed]
- Grandi P, Rybin V, Baßler J, et al. 90S Pre-Ribosomes Include the 35S Pre-rRNA, the U3 snoRNP, and 40S Subunit Processing Factors but Predominantly Lack 60S Synthesis Factors. Mol Cell 2002;10:105-15. [Crossref] [PubMed]
- Ebersberger I, Simm S, Leisegang MS, et al. The evolution of the ribosome biogenesis pathway from a yeast perspective. Nucl Acids Res 2014;42:1509-23. [Crossref] [PubMed]
- Eschrich D, Buchhaupt M, Kötter P, et al. Nep1p (Emg1p), a novel protein conserved in eukaryotes and archaea, is involved in ribosome biogenesis. Curr Genet 2002;40:326-38. [Crossref] [PubMed]
- Meyer B, Wurm JP, Kötter P, et al. The Bowen-Conradi syndrome protein Nep1 (Emg1) has a dual role in eukaryotic ribosome biogenesis, as an essential assembly factor and in the methylation of Ψ1191 in yeast 18S rRNA. Nucl Acids Res 2011;39:1526-37. [Crossref] [PubMed]
- Kornprobst M, Turk M, Kellner N, et al. Architecture of the 90S Pre-ribosome: A Structural View on the Birth of the Eukaryotic Ribosome. Cell 2016;166:380-93. [Crossref] [PubMed]
- Warda AS, Freytag B, Haag S, et al. Effects of the Bowen-Conradi syndrome mutation in EMG1 on its nuclear import, stability and nucleolar recruitment. Hum Mol Genet 2016;25:5353-64. [PubMed]
- Clevers H, Nusse R. Wnt/β-Catenin Signaling and Disease. Cell 2012;149:1192-205. [Crossref] [PubMed]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Development 2006;20:1394-404. [Crossref] [PubMed]
- Sinnberg T, Menzel M, Ewerth D, et al. β-Catenin Signaling Increases during Melanoma Progression and Promotes Tumor Cell Survival and Chemoresistance. PLoS One ;6:e23429. [Crossref] [PubMed]
- Xing Y, Clements WK, Kimelman D, et al. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev 2003;17:2753-64. [Crossref] [PubMed]
- Yin D, Li Y, Lin H, et al. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology 2013;24:105102. [Crossref] [PubMed]
- Benoit YD, Borhane G, Boyd AL, et al. Molecular pathways: epigenetic modulation of Wnt-glycogen synthase kinase-3 signaling to target human cancer stem cells. Clin Cancer Res 2014;20:5372-8. [Crossref] [PubMed]
- Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol 2013;5:a007898. [Crossref] [PubMed]
- Morgan RG, Jenna R, Alex T, et al. Factors affecting the nuclear localization of β-catenin in normal and malignant tissue. J Cell Biochem 2014;115:1351-61. [Crossref] [PubMed]


