
Antitumor activity and safety of sirolimus for solid tumors with PIK3CA mutations: A multicenter, open-label, prospective single-arm study (KM 02-01, KCSG UN17-16)
Introduction
Phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK) has important roles in cell growth and survival via the phosphatidylinositide 3-kinase (PI3K)-AKT serine/threonine kinase (AKT)-mammalian target of rapamycin kinase (mTOR) signaling cascades (1). Mutations in PIK3 catalytic subunit-alpha (PIK3CA), most frequently observed in the exon 9 helical domain (E542K and E545K) or exon 20 kinase domain (H1047R), can activate key proliferation signals in downstream pathways and contribute to tumor proliferation and survival (2-4). PIK3CA mutations or amplifications are frequently observed (14–36%) (5-8) and contribute to resistance to antitumor agents in solid tumors, such as cisplatin resistance in cervical cancer (9), HER2 resistance in breast cancer (10,11), or cetuximab resistance in colorectal cancer (12).
Numerous drugs targeting the PIK3-AKT-mTOR pathway have been developed (13-17). The first-generation inhibitors of this pathway were mTOR inhibitors, also known as rapamycin analogs (18). Sirolimus, originally developed as an anti-fungal agent (19), has antitumor activity that is exerted via binding to an intracellular protein (FKBP12), resulting in mTOR pathway inhibition and cell cycle arrest in the G1 phase (19,20). It has shown promising antitumor activity alone and in combination with other antitumor agents in preclinical (11,21) and clinical trials (22,23).
Based on the results of our phase I study that sirolimus has modest efficacy in advanced cancer with PIK3CA mutations/amplifications (24), we conducted a phase II study of the antitumor efficacy and safety of sirolimus in chemotherapy-refractory solid tumors with PIK3CA mutations.
Methods
Study design and treatment
A multi-center, open-label, single-arm prospective pilot study was conducted to evaluate the antitumor efficacy and safety of sirolimus in patients with solid tumors harboring PIK3CA mutations or amplifications. The study was conducted at 7 university hospitals in South Korea. Institutional Review Boards approved the protocol at all sites. All patients provided written informed consent. All study procedures followed the guidelines of the Declaration of Helsinki.
Oral sirolimus (1 mg daily) was administered continuously in 28-day cycles until disease progression, occurrence of unacceptable toxicity, patient’s request, or death due to any cause. The response was evaluated every 2 cycles from the start of the treatment based on the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). The primary endpoints were overall response rate (ORR) and safety. The secondary endpoints were disease control rate (DCR) and progression-free survival (PFS). All cases of toxicity were reviewed according to the National Cancer Institute Common Terminology Criteria of Adverse Events (CTCAE), version 4.03.
Patient eligibility
Eligible patients, 19 years of age or older, with pathologically confirmed solid tumors, who had undergone standard chemotherapy and had a confirmed PIK3CA mutation or amplification, as assessed by gene sequencing (CancerSCANTM or AxenTM) (24), were included. Further, patients were required to have at least one measurable or non-measurable lesion as per the revised response evaluation criteria in solid tumors (RECIST) v.1.1. (this lesion was evaluated at least 28 days before administration), Eastern Cooperative Oncology Group performance status 2 or lower, adequate hepatic [total bilirubin less than or equal to one and a half times the upper limit of normal (ULN)], aminotransferase (less than or equal to two and a half times the ULN), renal (adequate renal serum creatinine less than or equal to one and a half times the ULN), and hematologic (absolute neutrophil count of ≥1,500 cells/mL, platelet count of ≥75,000 cells/mL, and hemoglobin of ≥9 grams/dL) functions. Patients were also required to have appropriate heart function, without any clinically significant dysfunction that would require normal or medical intervention, as assessed by a 12-lead ECG and other history. All men and women of childbearing age had to use appropriate contraception.
Exclusion criteria were as follows: patients diagnosed with a malignant tumor other than a non-resistant solid tumor or a properly treated baseline or squamous cell skin cancer, history of hydrostatic or brain transfer (if the condition was previously treated properly and the patient was not currently receiving steroids as an anticonvulsant or for brain pressure control), and clinically significant gastrointestinal disorders that may cause a disturbance in the ingestion, distribution, or absorption of test drugs, such as that in the absence of the oral administration of purified water. Cardiovascular conditions within the last 6 months (cardiovascular surgery, myocardial infarction, unstable angina, coronary artery bypass surgery, and peripheral cardiovascular disease), uncoordinated convulsions, and central nervous system disease or combined psychiatric illness, which would make it difficult for the subject to voluntarily agree to a clinical trial. Women with children were required to stop breastfeeding before the first dose of the test medication until 14 days after the last dose. Patients with active infections or other uncontrolled disease and those previously administered sirolimus were also excluded.
Statistical analysis
ORR was defined as the proportion of patients with a complete (CR) or partial response (PR) to sirolimus. DCR was defined as the proportion of patients with CR, PR, or stable disease (SD) in response to the treatment. PFS was calculated using the Kaplan-Meier method from the time of sirolimus treatment to disease progression, patient’s request, or death due to any cause. Two-tailed P-values of <0.05 were considered significant. All analyses were performed using SPSS ver. 25.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
From July 2017 to March 2019, 24 patients with solid tumors harboring a PIK3CA mutation or amplification were treated with sirolimus. Most patients were female (66.6%) and had stage IV cancer (79.1%) at initial diagnosis. The median age at the initiation of sirolimus treatment was 57 years (range, 36–73 years). The most common type of cancer was colorectal cancer (29.1%), followed by sarcoma (16.6%), ovarian (12.5%), breast (8.3%), and lung cancer (8.3%), and others (cholangiocarcinoma, gallbladder, adrenal cortical, and stomach cancer, and unknown origin, 4.1% each).
H1047R and E545K were the most frequent PIK3CA mutations (16.6%), followed by amplification (12.5), E453K (8.3%), and E542K (8.3%). All patients had at least one second line of chemotherapy before sirolimus (median 4, range, 2 to 12). Patient characteristics are summarized in Table 1.
Table 1
| Patient number | Sex | Age | Histology | Stage at diagnosis | PIK3CA mutation | Sirolimus CTx (line) | Best Response | PFS (weeks) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 54 | Breast | I | H1047R | 10 | PD | 1.87 | |
| 2 | F | 72 | Sarcoma | IV | R818H | 5 | SD | 2.53 | |
| 3 | F | 58 | Colorectal | IV | H1047R | 6 | PD | 1.27 | |
| 4 | F | 45 | Gallbladder | IV | Q546K | 3 | PD | 1.83 | |
| 5 | M | 73 | Cholangiocarcinoma | IV | E453K | 3 | NA | 1.80 | Expired prior to disease evaluation |
| 6 | F | 57 | MUO | Unknown | E545K | 9 | PD | 2.67 | |
| 7 | M | 37 | Lung | IV | G545L | 4 | PD | 0.60 | |
| 8 | F | 42 | GBM | Unknown | R38S | 4 | SD | 5.03 | Ongoing |
| 9 | F | 57 | Ovarian | IV | E493X | 10 | NA | 0.97 | Non-target lesion |
| 10 | M | 57 | Stomach | IV | E453K | 5 | PD | 2.00 | |
| 11 | F | 56 | Colorectal | IV | E542K | 5 | PD | 2.23 | |
| 12 | F | 48 | Colorectal | IV | H1047R | 4 | PD | 1.97 | |
| 13 | M | 61 | Lung | IV | Amp | 4 | NA | 1.10 | Expired prior to disease evaluation |
| 14 | F | 68 | Sarcoma | IV | E545K | 3 | SD | 3.87 | |
| 15 | F | 59 | Colorectal | IV | E365K | 4 | PD | 1.73 | |
| 16 | F | 57 | Breast | III | Amp | 3 | SD | 3.93 | |
| 17 | M | 65 | Sarcoma | IV | Amp | 5 | PD | 1.90 | |
| 18 | F | 45 | Colorectal | IV | E545K | 4 | PD | 2.27 | |
| 19 | F | 36 | Ovarian | IV | E542K | 6 | NA | 0.70 | Withdrawal |
| 20 | M | 40 | Colorectal | III | E545K | 7 | PD | 2.40 | |
| 21 | M | 53 | Adrenal cortical | IV | G545A | 3 | PD | 2.53 | |
| 22 | M | 62 | Colorectal | IV | G106V | 5 | PD | 2.23 | |
| 23 | F | 50 | Sarcoma | IV | PTEN loss | 13 | SD | 8.83 | Ongoing |
| 24 | F | 55 | Ovarian | IV | H1047R | 8 | SD | 2.80 |
CTx, chemotherapy; MUO, malignancy of unknown origin; GBM, glioblastoma multiforme; amp, amplification.
Efficacy
Of 24 enrolled patients, 4 patients were excluded from the final analysis. Two patients had rapid disease progression-related death prior to the response evaluation, one patient withdrew consent and refused further treatment after 2 weeks, and one had a non-target lesion only. For the remaining 20 patients, baseline tumor measurements and response evaluations were performed.
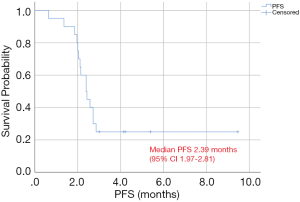
Evaluable patients received a median of 2.26 cycles of sirolimus during a median follow up time of 6.1 months. The ORR was 0% (none of the patients showed CR or PR), and DCR was 30% (6 SD) after sirolimus treatment. Median PFS was 2.39 months [95% confidence interval (CI), 1.97–2.81 months], and PFS at 8 weeks was 75% (Figure 1).
Among the patients who were eligible for the response evaluation, the mean volume change was 15.83%. SD was documented in 6 patients and PD in 14 patients. Tumor volume decreases (−12.08% to −13.88%) were observed in 4 patients with SD (20%). One patient was classified as PD owing to a new lesion, despite a decrease in the target lesion. The best response and absolute values for tumor control are summarized in Figure 2A.
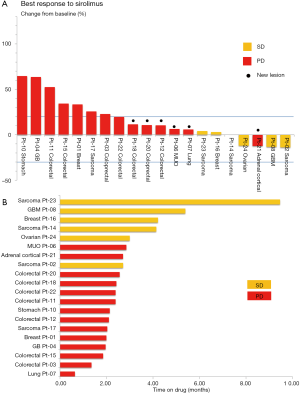
The median duration of response for patients with SD was 3.9 months (range, 2.53–8.83 months). The median duration for patients with PD was 2.2 months (range, 0.6–2.6 months). A swimmer plot of the sirolimus treatment duration for 20 evaluable patients is presented in Figure 2B. As per multivariate analysis, there were no independent prognostic factors for PFS.
Safety
During the treatment duration [median, 1.98 months (0.57–9.07 months)], 36 treatment-related adverse events were observed in the total population (n=24). Abdominal pain was the most common adverse event (20.8%), followed by fatigue (16.7%) and anemia (16.7%). In terms of severe adverse events (grade 3 or higher), anemia was the most common (16.7%), followed by fatigue, abdominal pain, and anorexia (8.3% each). No adverse events resulted in the discontinuation of treatment. The safety profile for the total population is provided in Table 2.
Table 2
| Adverse events | Any grade | Grade ≥3 | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Anemia | 4 | 16.7 | 4 | 16.7 | |
| Thrombocytopenia | 1 | 4.2 | 1 | 4.2 | |
| Neutropenia | 1 | 4.2 | 1 | 4.2 | |
| Fatigue | 4 | 16.7 | 2 | 8.3 | |
| Abdominal pain | 5 | 20.8 | 2 | 8.3 | |
| Anorexia | 3 | 12.5 | 2 | 8.3 | |
| Nausea | 2 | 8.3 | 0 | 0 | |
| Constipation | 1 | 4.2 | 0 | 0 | |
| Ascites | 1 | 4.2 | 1 | 4.2 | |
| Rash | 1 | 4.2 | 0 | 0 | |
| Dry skin | 1 | 4.2 | 0 | 0 | |
| Confusion | 1 | 4.2 | 1 | 4.2 | |
| Fever | 2 | 8.3 | 0 | 0 | |
| Sore throat | 2 | 8.3 | 0 | 0 | |
| Dyspnea | 1 | 4.2 | 0 | 0 | |
| Pleural effusion | 1 | 4.2 | 1 | 4.2 | |
| Urinary tract infection | 2 | 8.3 | 0 | 0 | |
| Upper respiratory infection | 2 | 8.3 | 0 | 0 | |
| Duodenal hemorrhage | 1 | 4.2 | 1 | 4.2 | |
Discussion
We comprehensively evaluated the clinical antitumor efficacy and safety of sirolimus monotherapy for patients with refractory solid tumors harboring PIK3CA aberrations. The efficacy of mTOR inhibitors for the treatment of advanced cancers is controversial (25-31). mTOR inhibitor monotherapy in pancreatic neuroendocrine tumors showed a survival benefit compared with a placebo group (11.0 vs. 4.6 months, P<0.001) (26). Temsirolimus single-agent therapy for advanced non-small cell lung cancer showed modest clinical activity (median PFS, 2.3–2.7 months) (30,31). However, mTOR inhibitor monotherapy in patients with gastric cancer was ineffective, with an ORR of 0% and PFS of 2.7 months in a phase II trial (27) and a PFS of 1.7 months in a phase III trial (28). Similar results were obtained for breast cancer (with a reported median TTP of 3 months) (29). However, patient selection in these studies did not consider biomarkers, such as PIK3CA-AKT-mTOR mutations.
Studies of genetic alterations in the PIK3CA-AKT-mTOR pathway have also yielded controversial results. Everolimus monotherapy in pS6 (Ser240/4)-mutated gastric cancer was associated with a prolonged PFS (2.76 vs. 1.57 months, P<0.001) (32). In the MOSCATO 01 trial, therapies targeting genetic alterations, including PIK3CA mutations, improved clinical outcomes (33). However, in a trial of molecularly targeted therapy based on tumor molecular profiling versus conventional therapy for advanced cancer (SHIVA), everolimus failed to show a significant benefit with regard to PFS compared to conventional therapy (median PFS 2.4 vs. 1.9 months, P=0.30) (34).
Notably, one patient with metastatic sarcoma with no response to at least eight regimens, including doxorubicin and ifosfamide, gemcitabine, pazopanib, and nivolumab, demonstrated long-term SD for >12 months. Consistent with these results, the phase III SUCCEED trial demonstrated a significantly lower risk of progression (by 28%) in the deforolimus (ridaforolimus, MK-8669) maintenance arm than in the placebo group (hazard ratio =0.72, P<0.0001) (35). Patients with sarcoma often harbor mutations in the mTOR signaling pathway, which are related to the antitumor activity of deforolimus in phase I and phase II trials when administered as maintenance therapy. Sirolimus is widely used as an immunosuppressive agent following transplantation. In post-transplant patients, sirolimus causes the regression of Kaposi’s sarcoma and renal angiomyolipomas in tuberous sclerosis (36,37). As the patent for the anti-cancer application of sirolimus expired in 1993, other rapalogs, such as temsirolimus, everolimus, and deforolimus have been developed (36).
Most recently, nab-sirolimus (ABI-009) has been developed as an albumin-bound mTOR inhibitor to increase tumor intake. A phase II trial of ABI-009 for advanced malignant perivascular epithelioid cell tumors (PEComa) demonstrated an ORR of 42% (38). Hence, the role of rapalogs as anti-cancer treatment for refractory cancer is a focus of current research.
Despite the insufficient efficacy of monotherapies, there is evidence that the use of mTOR inhibitors in combination with other drugs could provide a clinical benefit. The combination of sirolimus and pemetrexed exhibited meaningful antitumor activity in pretreated advanced non-small cell lung cancer (23). The combination of trastuzumab plus vinorelbine significantly prolonged PFS in trastuzumab-resistant HER2-positive breast cancer (39). Collectively, mTOR inhibitors are more effective for tumor stabilization than reduction. The use mTOR inhibitors in combination with other antitumor agents might have synergistic effects.
However, our study had several limitations. The relatively small number of patients and heterogeneity of cancer types could preclude a precise analysis of the association between PIK3CA mutations and the efficacy of sirolimus therapy. The heavily pre-treated nature of the patients could indicate a high mutation burden in tumors, suggesting the co-existence of other tumorigenic genetic alterations. Finally, sirolimus only inhibits mTORC1 activity and not the mTORC2-mediated cascade; accordingly, it only partially inhibits the mTOR pathway. Considering the complicated signaling cascade and various cross signaling reactions with other pathways, sirolimus alone was not sufficient to evaluate the true association between PIK3CA mutation or amplification and mTOR inhibitor efficacy.
Recently developed next-generation mTOR inhibitors blocking multiple targets in the PIK3CA-AKT-mTOR pathway show promising efficacy in various tumors (40-42). Further studies are needed to establish the roles of mTOR inhibitors in individualized cancer treatment and validate the prognostic value of biomarkers.
Acknowledgments
Funding: This research was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.07). SB reports grants from Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), grants from Ministry of Health & Welfare, Republic of Korea, non-financial support from Korean Cancer Study Group and KCSG data center, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional Review Boards approved the protocol at all sites (No. SMC 2016-02-052-040). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006;441:424-30. [Crossref] [PubMed]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489-501. [Crossref] [PubMed]
- Regad T. Targeting RTK Signaling Pathways in Cancer. Cancers (Basel) 2015;7:1758-84. [Crossref] [PubMed]
- Shin MK, Payne S, Bilger A, et al. Activating Mutations in Pik3ca Contribute to Anal Carcinogenesis in the Presence or Absence of HPV-16 Oncogenes. Clin Cancer Res 2019;25:1889-900. [Crossref] [PubMed]
- Cousin S, Grellety T, Toulmonde M, et al. Clinical impact of extensive molecular profiling in advanced cancer patients. J Hematol Oncol 2017;10:45. [Crossref] [PubMed]
- Rodon J, Dienstmann R, Serra V, et al. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 2013;10:143-53. [Crossref] [PubMed]
- Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014;506:371-5. [Crossref] [PubMed]
- Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333-9. [Crossref] [PubMed]
- Arjumand W, Merry CD, Wang C, et al. Phosphatidyl inositol-3 kinase (PIK3CA) E545K mutation confers cisplatin resistance and a migratory phenotype in cervical cancer cells. Oncotarget 2016;7:82424-39. [Crossref] [PubMed]
- Wang L, Zhang Q, Zhang J, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer 2011;11:248. [Crossref] [PubMed]
- Elkabets M, Vora S, Juric D, et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med 2013;5:196ra99. [Crossref] [PubMed]
- Xu JM, Wang Y, Wang YL, et al. PIK3CA Mutations Contribute to Acquired Cetuximab Resistance in Patients with Metastatic Colorectal Cancer. Clin Cancer Res 2017;23:4602-16. [Crossref] [PubMed]
- Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012;30:282-90. [Crossref] [PubMed]
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009;27:3822-9. [Crossref] [PubMed]
- Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol 2012;30:777-82. [Crossref] [PubMed]
- Lopez S, Schwab CL, Cocco E, et al. Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol 2014;135:312-7. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets 2008;12:209-22. [Crossref] [PubMed]
- Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003;35:7S-14S. [Crossref] [PubMed]
- Vilella-Bach M, Nuzzi P, Fang Y, et al. The FKBP12-rapamycin-binding domain is required for FKBP12-rapamycin-associated protein kinase activity and G1 progression. J Biol Chem 1999;274:4266-72. [Crossref] [PubMed]
- De Martino MC, Feelders RA, Pivonello C, et al. The role of mTOR pathway as target for treatment in adrenocortical cancer. Endocr Connect 2019;8:R144-56. [Crossref] [PubMed]
- Rizell M, Andersson M, Cahlin C, et al. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol 2008;13:66-70. [Crossref] [PubMed]
- Komiya T, Memmott RM, Blumenthal GM, et al. A phase I/II study of pemetrexed with sirolimus in advanced, previously treated non-small cell lung cancer. Transl Lung Cancer Res 2019;8:247-57. [Crossref] [PubMed]
- Jung KS, Lee J, Park SH, et al. Pilot study of sirolimus in patients with PIK3CA mutant/amplified refractory solid cancer. Mol Clin Oncol 2017;7:27-31. [Crossref] [PubMed]
- de Fijter JW. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation 2017;101:45-55. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [Crossref] [PubMed]
- Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 2010;28:1904-10. [Crossref] [PubMed]
- Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935-43. [Crossref] [PubMed]
- Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 2005;23:5314-22. [Crossref] [PubMed]
- Reungwetwattana T, Molina JR, Mandrekar SJ, et al. Brief report: a phase II "window-of-opportunity" frontline study of the MTOR inhibitor, temsirolimus given as a single agent in patients with advanced NSCLC, an NCCTG study. J Thorac Oncol 2012;7:919-22. [Crossref] [PubMed]
- Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol 2009;20:1674-81. [Crossref] [PubMed]
- Yoon DH, Ryu MH, Park YS, et al. Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum. Br J Cancer 2012;106:1039-44. [Crossref] [PubMed]
- Massard C, Michiels S, Ferte C, et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov 2017;7:586-95. [Crossref] [PubMed]
- Belin L, Kamal M, Mauborgne C, et al. Randomized phase II trial comparing molecularly targeted therapy based on tumor molecular profiling versus conventional therapy in patients with refractory cancer: cross-over analysis from the SHIVA trial. Ann Oncol 2017;28:590-6. [Crossref] [PubMed]
- Chawla SP, Blay J, Ray-Coquard IL, et al. Results of the phase III, placebo-controlled trial (SUCCEED) evaluating the mTOR inhibitor ridaforolimus (R) as maintenance therapy in advanced sarcoma patients (pts) following clinical benefit from prior standard cytotoxic chemotherapy (CT). J Clin Oncol 2011;29:10005. [Crossref]
- Kolhe N, Mamode N, Van der Walt J, et al. Regression of post-transplant Kaposi's sarcoma using sirolimus. Int J Clin Pract 2006;60:1509-12. [Crossref] [PubMed]
- Krischock L, Beach R, Taylor J. Sirolimus and tuberous sclerosis-associated renal angiomyolipomas. Arch Dis Child 2010;95:391-2. [Crossref] [PubMed]
- Wagner AJ, Ravi V, Ganjoo KN, et al. ABI-009 (nab-sirolimus) in advanced malignant perivascular epithelioid cell tumors (PEComa): Preliminary efficacy, safety, and mutational status from AMPECT, an open label phase II registration trial. J Clin Oncol 2019;37:11005. [Crossref]
- Andre F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [Crossref] [PubMed]
- Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today 2011;16:325-31. [Crossref] [PubMed]
- Bendell JC, Varghese AM, Hyman DM, et al. A First-in-Human Phase 1 Study of LY3023414, an Oral PI3K/mTOR Dual Inhibitor, in Patients with Advanced Cancer. Clin Cancer Res 2018;24:3253-62. [Crossref] [PubMed]
- Ippen FM, Alvarez-Breckenridge CA, Kuter BM, et al. The Dual PI3K/mTOR Pathway Inhibitor GDC-0084 Achieves Antitumor Activity in PIK3CA-Mutant Breast Cancer Brain Metastases. Clin Cancer Res 2019;25:3374-83. [Crossref] [PubMed]


