The coexistence of recurrent pituitary adenoma and meningioma: case report
Introduction
Pituitary adenoma is a common benign tumour in the brain. Recently, its incidence has increased annually, accounting for 15–25% of all intracranial tumours (1). Meningioma is another common intracranial tumour that accounts for 15–25% of all central nervous system neoplasms (2,3). However, the coexistence of pituitary adenoma and meningioma is extremely rare and is even rarer in patients with no previous history of irradiation. Here, we present a case of recurrent non-functioning pituitary adenoma and left temporal lobe meningioma in a patient without a previous history of irradiation. As far as we know, this appears to be the first description of the coexistence of recurrent non-functioning pituitary adenoma and meningioma in a patient with no previous history of irradiation. We present the following case in accordance with the CARE Guideline (4).
Case presentation
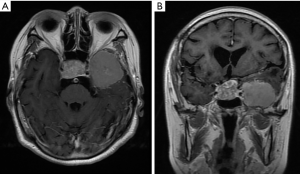
A 73-year-old woman underwent a right-sided craniotomy of a saddle region tumour to treat visual impairment in 1987. Her daughter recalled that the histological diagnosis was a non-functioning pituitary adenoma. However, the medical record and imaging data were missing. The patient was discharged uneventfully without irradiation. She presented again in May 2018 with a two-year history of lack of alertness, speech confusion and visual impairment. She had no other relevant medical history, and she did not smoke or consume alcohol. Therefore, a brain magnetic resonance imaging (MRI) scan was performed. The brain MRI revealed a recurrent pituitary adenoma and a left temporal lobe tumour (Figure 1).
Her preoperative hormone blood levels were as follows: total T3, 0.87 nmol/L (reference range, 1.30–3.10 nmol/L); total T4, 48.52 nmol/L (reference range, 66.00–181.00 nmol/L); free T3, 2.80 pmol/L (reference range, 2.80–7.10 pmol/L); free T4, 7.59 pmol/L (reference range, 11.46–23.17 pmol/L); thyroid stimulating hormone (TSH), 4.11 µIU/mL (reference range, 0.30–5.50 µIU/mL); luteinizing hormone (LH), 3.63 mIU/L (reference range, 10.87–58.64 IU/L in postmenopausal women); follicle-stimulating hormone (FSH), 10.18 mIU/mL (reference range, 16.74–113.59 mIU/mL in postmenopausal women); prolactin (PRL), 16.74 mg/mL (reference range, 2.74–19.64 mg/mL in postmenopausal women); and growth hormone (GH), 0.22 ng/mL (reference range, 0.06–5 ng/mL). Neurological examination showed that the uncorrected visual acuity was 0.2 in the left eye and 0.3 in the right eye; visual field examination revealed patchy defects.
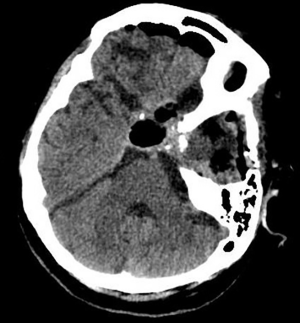
After discussion with the patient and the patient’s family members, the decision was made to resect the recurrent pituitary adenoma and the left temporal lobe tumour simultaneously. The patient underwent a left frontotemporal craniotomy, and total removal of the temporal lobe tumour and pituitary adenoma was achieved using a microsurgical technique. The postoperative computed tomography scan performed 6 hours after the surgery showed no evidence of residual tumours (Figure 2).
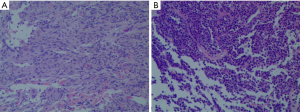
The histology of the saddle region tumour revealed that it was a pituitary adenoma, and the immunohistochemical results were as follows: Ki-67 (1%+), Syn (+), CgA (+), CK8/18 (−), adrenocorticotropic hormone (ACTH) (−), GH (−), PRL (−), FSH (partly +), LH (−), and TSH (−) (Figure 3A). The histology of the temporal lobe tumour revealed that it was a meningioma of transitional type (Figure 3B).
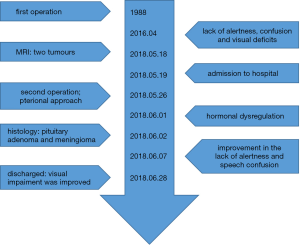
After the surgery, the patient showed improvement in her lack of alertness and speech confusion. The visual impairment was slightly improved. Postoperatively, the patient was found to have hormonal dysregulation and needed thyroid hormone replacement therapy. She was discharged with no significant neurological deficits. The timeline picture of the patient was shown in Figure 4.
Discussion
The coexistence of a pituitary adenoma and an intracranial meningioma is a very rare event (5,6), especially in patients with a history of pituitary adenoma and without a history of previous irradiation (7). Among the reported cases of coexistent pituitary adenoma and meningioma, planum sphenoidale, tuberculum sellae and the sphenoid wing meningiomas clearly predominate (5,7).
The aetiology of coexistent pituitary adenoma and intracranial meningioma is unknown. Coexistent meningiomas have been reported in patients with non-functioning pituitary adenoma, prolactinoma and Cushing disease after radiotherapy (8-10), but the coexistence of meningioma and these types of pituitary tumour has also been described in patients who were not previously irradiated (11), suggesting that the coexistence of meningioma and pituitary adenoma may not imply a relationship between the two diseases. Certain hormones, such as oestrogens and prolactin, are recognised to have roles in stimulating the growth of meningiomas (12). In general, prolactinomas are the most common pituitary adenomas, but GH-producing tumours are the most commonly secreting adenomas that are found co-occurring with meningiomas (7).This evidence indicates that GH or somatostatin may stimulate the dura and arachnoid cells and may play roles in the occurrence or growth of meningioma (13); however, this statement has yet to be proven. In addition,it is possible that genetic alterations shared by these two tumours on the same chromosomes may explain their simultaneous occurrence (14-16).
The coexistent pituitary adenoma and intracranial meningioma in one patient presented a surgical and management challenge. In most previous reported cases, the coexistent pituitary adenoma and intracranial meningioma were managed independently, usually involving addressing the pituitary adenoma with a transsphenoidal approach and treating the meningioma separately with conservative measures or another surgical approach. When the pituitary adenoma and meningioma are contiguous, they can be removed in a one-stage operation using a single pterional approach or an endoscopic expanded endonasal approach. Compared to the surgical treatment of a single pituitary adenoma or meningioma, any of these surgical approaches have significant increases in the level of risk involved. Thus, adequate knowledge of the coexistent pituitary adenoma and meningioma is a very important precondition to planning the appropriate surgical approach and avoiding severe surgical complications.
Here, we present a case of recurrent non-functioning pituitary adenoma and temporal lobe meningioma in a patient without previous irradiation. To our knowledge, this is the first description of the coexistence of recurrent non-functioning pituitary adenoma and meningioma in a patient with no previous history of irradiation. In this case, we resected the recurrent pituitary adenoma and the meningioma simultaneously. The simultaneous removal of two tumours carries a higher risk than resection of the pituitary tumour and meningioma in two stages. Finally, the patient was discharged with no significant neurological deficits. There are two limitations to this study. First, the medical records and imaging data for the first operation were missing. Second, as similar cases are rare, the aetiology of coexistent pituitary adenoma and meningioma is unknown.
Conclusions
In conclusion, coexistent pituitary adenoma and temporal lobe meningioma is a very rare surgical entity, and diagnosis poses a therapeutic challenge. In this case, we used a single pterional approach for both tumours. The results prove that the treatment is feasible. The aetiology of coexistent pituitary adenoma and intracranial meningioma is unknown, and more cases and additional studies are necessary to explain such unusual findings.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.78). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raappana A, Koivukangas J, Ebeling T, et al. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab 2010;95:4268-75. [Crossref] [PubMed]
- Karsy M, Guan J, Cohen A, et al. Medical Management of Meningiomas: Current Status, Failed Treatments, and Promising Horizons. Neurosurg Clin N Am 2016;27:249-60. [Crossref] [PubMed]
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery 2005;57:1088-95. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Honegger J, Buchfelder M, Schrell U, et al. The coexistence of pituitary adenomas and meningiomas: three case reports and a review of the literature. Br J Neurosurg 1989;3:59-69. [Crossref] [PubMed]
- Cannavo S, Curto L, Fazio R, et al. Coexistence of growth hormone-secreting pituitary adenoma and intracranial meningioma: a case report and review of the literature. J Endocrinol Invest 1993;16:703-8. [Crossref] [PubMed]
- Prevedello DM, Thomas A, Gardner P, et al. Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: technical case report. Neurosurgery 2007;60:E401; discussion E401.
- Spallone A. Meningioma as a sequel of radiotherapy for pituitary adenoma. Neurochirurgia (Stuttg) 1982;25:68-72. [PubMed]
- Kolodny J, Dluhy RG. Recurrent prolactinoma and meningioma following irradiation and bromocriptine treatment. Am J Med 1985;78:153-5. [Crossref] [PubMed]
- Partington MD, Davis DH. Radiation-induced meningioma after treatment for pituitary adenoma: case report and literature review. Neurosurgery 1990;26:329-31. [Crossref] [PubMed]
- Curto L, Squadrito S, Almoto B, et al. MRI finding of simultaneous coexistence of growth hormone-secreting pituitary adenoma with intracranial meningioma and carotid artery aneurysms: report of a case. Pituitary 2007;10:299-305. [Crossref] [PubMed]
- Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010;99:307-14. [Crossref] [PubMed]
- De Menis E, Tulipano G, Villa S, et al. Development of a meningioma in a patient with acromegaly during octreotide treatment: are there any causal relationships? J Endocrinol Invest 2003;26:359-63. [Crossref] [PubMed]
- Ben Nsir A, Khalfaoui S, Hattab N. Simultaneous Occurrence of a Pituitary Adenoma and a Foramen Magnum Meningioma: Case Report. World Neurosurg 2017;97:748.e1-748.e2. [Crossref] [PubMed]
- Pravdenkova S, Al-Mefty O, Sawyer J, et al. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg 2006;105:163-73. [Crossref] [PubMed]
- Bello MJ, de Campos JM, Kusak ME, et al. Chromosomal abnormalities in pituitary adenomas. Cancer Genet Cytogenet 2001;124:76-9. [Crossref] [PubMed]