
Secondary malignancies after radiation therapy in prostate cancer survivors: a propensity-score matched competing-risk analysis
Introduction
An estimate of 164,690 new prostate cancer (Pca) cases were diagnosed in 2018 and 29,430 deaths from this condition were also reported. Pca accounts for 9.5% of all new cancer cases and 4.8% of overall mortality (1). Despite the high prevalence of this disease, cancer-specific survival is excellent in most patients (1,2). The mortality rate is falling thanks to advances in medical technology (3).
However, cancer survivors are susceptible to developing secondary malignancies due to intrinsic genetic factors, lifestyle (tobacco smoking, obesity, physical inactivity or excessive sun exposure), and potential treatment carcinogenicity [radiotherapy (RT), chemotherapy, or hormonal therapy]. Several studies have demonstrated secondary malignancies after therapeutic radiation in various cancer types (4-7). However, data linking RT to Pca, as well as subsequent malignancy, are still unclear (8-11). Previous studies focusing on this topic often neglect the choice of most beneficiaries. Accurately defining the patient population who are candidates for RT is critical to prevent secondary malignancies. Usually, early-stage, low-grade localized Pca survivors aged ≤75 years are the most susceptible to RT-induced secondary malignancies. By bringing to light this controversial issue, we hope physicians can provide an adequate precision surveillance strategy for Pca survivors.
Methods
Study population
This analysis followed the Declaration of Helsinki and was exempt from ethics approval. Based on the Surveillance, Epidemiology, and End Results database (SEER), we conducted a study of Pca survivors between 2004–2013. Pca survivors are defined as men who have been diagnosed with prostate adenocarcinoma (ICD-O-3: 8140/3) and have undergone a single course of initial treatment, such as radical prostatectomy (RP), RT, or in the case of one group, no active treatment. RP included the open retropubic procedure and as well as minimally invasive methods. RT included external beam, brachytherapy, or a combination of both. The group that did not receive any active treatment underwent what we refer to as expectant or conservative management, which involved two treatment modalities: active surveillance (AS), and watchful waiting (WW). We selected Pca candidates aged ≤75 years who were diagnosed with stage T1 to T2 cancer, and had a Gleason score (GS) of 6 to 7 (Gleason grade group 1–2), and did not have any nodal involvement or distant metastasis (N0/M0).
To accurately define the appropriate treatment recommendation for different populations, we further divided this cohort into a low-risk group (T1 to T2a, GS =6, and prostate-specific antigen (PSA) <10 ng/mL), and an intermediate-risk group (T2b to T2c, or GS =7, or PSA 10 to 20 ng/mL), according to the National Comprehensive Cancer Network (NCCN) guidelines (12).
Secondary malignancies were assessed and reported 1 year after the initial diagnosis of Pca, with the end of the follow-up period being in December 2013. Secondary Pca was not counted as secondary malignancy. All-cause death was regarded as a competing event.
Statistical analysis
Variables were presented as medians (interquartile range, IQR). Mann-Whitney U-test and Chi-square statistics were used to analyze the patient characteristics and proportions. P value adjusted for multiple comparisons was set at 0.0167. The cumulative incidence function regarding competing risks was applied to estimate the incidence of secondary malignancy among the three arms. Differences were calculated using the Gray’s test. The relationships between the risk of secondary malignancies and RT and the other two treatments were analyzed using the Fine-Gray regression models, considering death as competing risks. The sensitivity analysis was conducted using the Cox regression censoring death. Proportional hazard assumptions were visually checked using log-log plots.
To control selection bias and potential confounding, we performed a propensity-score matched analysis. Age, year of diagnosis, marital status, race, PSA, GS, and T stage were used in a logistic regression model to estimate the propensity-score. According to the estimated propensity-score, the RT group was matched in 1:1 ratio, with no replacement to the RP or no active treatment group; this was performed using the nearest-neighbor method with a 0.00001 caliper set. The Mann-Whitney U-test or Pearson chi-square test was applied to assess the post-match balance in the baseline covariates.
Baseline data analyses were performed using SPSS, version 23 (IBM, Chicago, IL, USA). Stata, version 12.0 (StataCorp, College Station, TX, USA), and R version 3.4.0 (The R Foundation for Statistical Computing, Vienna, Austria) were used for the Fine-Gray regression and propensity-score matching algorithm. The flow chart was illustrated with Review Manager, version 5.3 (Nordic Cochrane Center, Cochrane Collaboration, Copenhagen). Pie charts were drawn using GraphPad Prism, version 6.01 (GraphPad Software Incorporated, San Diego, CA, USA). A two-sided P value <0.05 was regarded as statistically significant in the Cox regression models.
Results
Patient characteristics
A total of 234,349 Pca patients were included in the study (Figure 1). The mean follow-up was 9.8 years (ranging from 1 to 9.9 years) and the median age was 63 years (IQR, 58–68 years). Forty-six thousand four hundred sixteen (19.8%) patients underwent no active treatment, whilst 100,020 (42.7%) underwent RP, and 87,913 (37.5%) underwent RT. Table 1 summarizes the characteristics of these three groups.
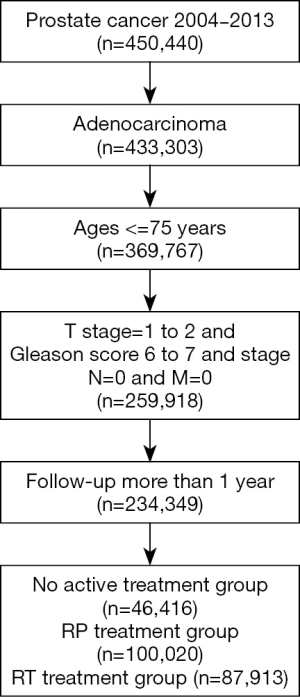
Table 1
| All patients | No active treatment | RP | RT | P valuea | P valueb | |
|---|---|---|---|---|---|---|
| Total, n (%) | 234,349 [100] | 46,416 (19.8) | 100,020 (42.7) | 87,913 (37.5) | ||
| Mean follow-up (years) | 9.8 | 9.7 | 9.8 | 9.7 | ||
| Age (years), median (IQR) | 63 [58–68] | 65 [59–70] | 60 [55–65] | 66 [60–70] | <0.001 | <0.001 |
| PSA (ng/ml), median (IQR) | 5.7 (4.4–7.9) | 5.7 (4.5–7.9) | 5.3 (4.3–7.2) | 6.1 (4.7–8.7) | <0.001 | <0.001 |
| Race, n (%) | 0.876 | <0.001 | ||||
| White | 181,975 (79.5) | 33,289 (76.8) | 82,179 (76.9) | 66,507 (83) | ||
| Black | 36,270 (15.9) | 7992 (18.4) | 12,397 (12.5) | 15,881 (18.4) | ||
| Other | 10,568 (4.6) | 2063 (4.8) | 4430 (4.5) | 4075 (4.7) | ||
| Marital status, n (%) | <0.001 | <0.001 | ||||
| Single | 22,884 (11) | 4,929 (14.9) | 9,117 (9.7) | 8,838 (11.1) | ||
| Married | 160,997 (77.6) | 23,250 (70.1) | 77,305 (81.9) | 60,442 (75.6) | ||
| Divorced/separated | 17,720 (8.5) | 3,840 (11.6) | 6,338 (6.7) | 7,542 (9.4) | ||
| Widowed | 5,881 (2.8) | 1,169 (3.5) | 1,628 (1.7) | 3,084 (3.9) | ||
| T stage, n (%) | <0.001 | <0.001 | ||||
| T1 | 91,451 [39] | 29,417 (63.4) | 612 (0.6) | 61,422 (69.9) | ||
| T2 | 142,898 [61] | 16,999 (36.6) | 99,408 (99.4) | 26,491 (30.1) | ||
| Gleason score, n (%) | <0.001 | <0.001 | ||||
| 6 | 131,297 [56] | 34,735 (74.8) | 46,354 (46.3) | 50,208 (57.1) | ||
| 7 | 103,052 [44] | 11,681 (25.2) | 53,666 (53.7) | 37,705 (42.9) | ||
| Year of diagnosis | <0.001 | <0.001 | ||||
| 2004–2006 | 69,478 (29.6) | 11,344 (24.4) | 28,765 (28.8) | 29,369 (33.4) | ||
| 2007–2009 | 84,822 (36.2) | 15,663 (33.7) | 37,476 (37.5) | 31,683 [36] | ||
| 2010–2012 | 80,049 (34.2) | 19,409 (41.8) | 33,779 (33.8) | 26,861 (30.6) | ||
| NCCN risk group | <0.001 | <0.001 | ||||
| Low-risk | 20,800 (13.5) | 8879 (41.8) | 2349 (2.8) | 9472 (19.8) | ||
| Intermediate-risk | 133,108 (86.5) | 12,478 (58.2) | 82,162 (97.2) | 38,468 (80.2) |
a, P value was determined between RT and no active treatment cohort; b, P value was determined between RT and RP cohort. IQR, interquartile range; PSA, prostate-specific antigen; Pca, prostate cancer; RP, radical prostatectomy; RT, radiation therapy; NCCN, The National Comprehensive Cancer Network.
Frequency of secondary malignancies
Four thousand two hundred twenty-two (1.8%) secondary malignancies were identified 1 year after the diagnosis of Pca, of which, 870 (1.9%) occurred in the no active treatment cohort, 1,241 (1.2%) occurred in RP cohort, and 2,117 (2.4%) occurred in the RT cohort (Table 2). As seen in Table S1, the five most common secondary malignancies were lung cancer (N=794, 18.8%), bladder cancer (N=520, 12.3%), colorectal cancer (N=465, 11.0%), melanoma (N=370, 8.8%), and non-Hodgkin lymphoma (N=245, 5.8%). The site-specific frequency is slightly different in the three arms, whilst lung cancer remains the most frequent; no active treatment (N=182, 20.9%), RP (N=176, 14.2%), and RT (N=436, 20.6%) (Figure 2).
Table 2
| Developed secondary malignancy | Alive | Death | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Percentage (%) | N | Percentage (%) | N | Percentage (%) | N | Percentage (%) | ||||
| The entire cohort | 4,228 | 1.8 | 219,237 | 93.6 | 10,884 | 4.6 | 23,4349 | 100 | |||
| No active treatment | 870 | 1.9 | 42,196 | 90.9 | 3,350 | 7.2 | 46,416 | 100 | |||
| RP | 1,241 | 1.2 | 96,890 | 96.9 | 1,889 | 1.9 | 100,020 | 100 | |||
| RT | 2,117 | 2.4 | 80,151 | 91.2 | 5,645 | 6.4 | 87,913 | 100 | |||
Pca, prostate cancer; RP, radical prostatectomy; RT, radiation therapy.
Cumulative incidence of secondary malignancies
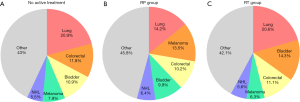
Figure 3 displays the cumulative incidence of secondary malignancy in the three treatments groups. After 10-year of follow-up, the risk of secondary malignancies were comparable in patients treated with RT and no active treatment (P=0.070). After 6 years of follow-up, the incidence curve for the RT group began to separate from the no active treatment group (Table S2). A significant difference in secondary malignancy risks was observed in RT versus RP (P<0.001) as there was an early separation of the incidence curves early from the beginning (Table S2).
Sensitivity analyses
In the competing-risk analysis, patients who underwent RT were associated with similar secondary malignancies risk compared with those who received no active treatment (HR =1.082; 95% CI: 1–1.171, P=0.051). According to Cox regression censoring death, there was no significant difference in secondary malignancies that could be observed in RT versus no active treatment (HR =1.059; 95% CI: 0.979–1.146, P=0.154). After propensity-score matching and weighting with adjustment for age, year of diagnosis, marital status, race, PSA, GS, and T stage, the findings were consistent (P=0.220 and 0.407). In the competing-risk analysis, patients who underwent RT were associated with more secondary malignancies than those who received RP (HR =1.846; 95% CI: 1.721–1.980, P<0.001). In the Cox regression censoring death, increased secondary malignancies can be observed in RT versus RP (HR =1.896; 95% CI: 1.767–2.033, P<0.001). After propensity-score adjusting, the findings were also equivalent (all P<0.001) (Table 3).
Table 3
| Unadjusted analysis | Propensity-adjusted analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (Events) | HR (95% CI) | P value | HR (95% CI)a | P valuea | N (Events) | HR (95% CI)b | P valueb | HR (95% CI)c | P valuec | ||
| RT vs. no active treatment (Ref.) | 134,329 [2,987] | 1.059 (0.979–1.146) | 0.154 | 1.082 (1–1.171) | 0.051 | 59,226 [1,439] | 1.045 (0.942–1.159) | 0.407 | 1.067 (0.962–1.183) | 0.220 | |
| RT vs. RP (Ref.) | 187,933 [3,358] | 1.896 (1.767–2.033) | <0.001 | 1.846 (1.721–1.980) | <0.001 | 43,260 [1,044] | 1.577 (1.393–1.786) | <0.001 | 1.539 (1.359–1.742) | <0.001 | |
a, competing-risk analysis; b, Propensity-score matching and weighting with adjustment for age, year of diagnosis, marital status, race, PSA, Gleason score, AJCC T stage; c, Propensity-score matching and weighting with adjustment for age, year of diagnosis, marital status, race, PSA, Gleason score, AJCC T stage for competing-risk analysis. Pca, prostate cancer; RP, radical prostatectomy; RT, radiation therapy; HR, hazard ratio; CI, confidence interval; PSA, prostate-specific antigen; AJCC, The American Joint Committee on Cancer.
Subgroup analyses
When stratifying with the NCCN risk group, we did not observe a difference in secondary malignancy risks for patients who underwent RT compared with those who received no active treatment (HR =1.081; 95% CI: 0.706–1.657, P=0.720), and RP (HR =1.316; 95% CI: 0.647–2.679, P=0.448) in the low-risk group. After a propensity-score adjustment, this trend persisted among patients in the RT group compared with patients who received no active treatment or RP (P=0.195 and 0.252). In the intermediate-risk group, an increased risk of secondary malignancies could be seen in patients who underwent RT compared with those who received RP (HR =2.110; 95% CI: 1.935–2.301, P<0.001). No difference was found in RT versus no active treatment (HR =1.047; 95% CI: 0.920–1.192, P=0.487). After the propensity-score adjustment, this tendency persisted among patients in the RT group compared with patients who received RP (P<0.001) or no active treatment (P=0.634) (Table S3).
Discussion
Our study showed that patients undergoing RT have a similar risk to develop a secondary malignancy compared with the no treatment group. Simultaneously, a significantly lower risk can be observed in their RP counterparts.
Accurate life expectancy estimation is crucial in Pca management. Young age Pca survivors tend to have a higher chance to develop a secondary malignancy due to their longer survival. Social Security Administration life tables can be used to estimate life expectancy in Pca patients, which have been endorsed by the NCCN guidelines (12-14). We selected Pca men who were diagnosed younger than ≤75 years because they comprise the majority of newly diagnosed with Pca and have a life expectancies of more than 10 years (13). Young Pca patients with few comorbidities might be more likely to balance the trade of life gained and harm, whereas older patients with other health risks, often do not factor this risk into their day to day decision making.
In a local disease with a long-life expectancy, no active treatment and definitive treatment (RP/RT) can be considered for Pca patients (15). The treatment choice is determined by patient preference, clinician judgment, and resource availability. Recently, Prostate Testing for Cancer and Treatment (ProtecT) trials provided high-quality evidence of treatment for men with clinically localized PSA-detected Pca (16,17). In a low- and intermediate-risk population, the ProtecT trial compared RP, RT, and active monitoring and found no difference in the cancer mortality at 10 years (16). This trial also showed that both RP and RT resulted in short-term adverse effects compared with the control group; specifically, worse urinary and sexual function after RP, and more bowel symptoms after RT. However, the gap was progressively decreasing and did not seem to differ among the three groups after 6 years of follow-up (17). Two reports published in JAMA also supported ProtecT results (18,19).
In terms of cancer mortality and patient-reported outcome, the three arms (RP, RT or AS) in a clinically localized Pca cohort have equally effective results. Therefore, other side-effects and comorbidities are essential in the decision-making process, such as the incidence of secondary malignancies. Previous studies that investigated radiation-induced second malignancies in Pca survivors have yielded ambiguous results. Leveraging a large-scale, population-based cohort, we hope to provide the best evidence regarding the safety of therapeutic radiation in Pca survivors.
The suitable time frame for radiation-related cancers development is constantly being debated. Previously, it has ranged from 1 to 10 years in Pca men (20,21). In a comprehensive review and meta-analysis, the results were consistent after adjusting for different lag time restrictions (11). In this study, we chose 1 year Pca survivors so that we could investigate radiation-related cancers, similar to other studies (21-23). Moreover, Pca survivors with low-grade or organ-confined diseases (GS 6–7, T1-T2) were selected as our study population. For tumors with high-grade or other aggressive features, an immediate and effective treatment should be given, regardless of how much risk of second malignancies there is. The NCCN risk groups are used to provide a better therapeutic recommendation and clinical decision making (12). We organized a subgroup analysis stratification based on the NCCN risk classification. Interestingly, there was no differences between RT versus RP in low-risk patients; moreover, the most significant differences were recorded in intermediate-risk patients, which may be due to the influence of RT dose and androgen deprivation.
Limitations
Our study has several limitations, hence the results should be interpreted with caution as it may have been influenced by inherent bias from the SEER database. Firstly, a comparison against the no active treatment group has limited value since this is not a homogenous group. The definition of AS or WW is not specified in the SEER data. WW is used to describe the approach to patients with a limited remaining lifetime. AS is the most likely choice in our no active treatment cohort. In previous AS studies, 30% to 50% of patients were treated 2 to 3 years after cancer diagnosis. This could not be specified in our study given the SEER database limitation, and it is very likely that these patients presented with comorbidities and no treatment were proposed. This most likely led to selection bias toward falsely lower estimations of secondary cancer incidence in the no treatment group.
The SEER database also provided limited information regarding patients’ comorbidities such as cardiovascular disease, pulmonary diseases as well as risk factors such as smoking status or obesity, which are all crucial when clinicians recommend appropriate treatment for Pca. The incidence curve of secondary cancer becomes immediately different between RT and RP, and consequently, the relevance of this work becomes questionable. Unbalanced health characteristics in the two groups may explain the inferior incidence seen with RP. Pca patients with fewer comorbidities may be more likely to receive RP. We should note that in our study, RP patients were younger and had lower PSA values at diagnosis. Age is a well-known risk factor for cancer, so elderly patients (those treated with RT) tend to have a higher risk of cancer after Pca therapy. Although a propensity-score matched analysis adjusted for age, PSA, GS, and T stage was performed to control selection bias, we may need to provide more details on what exactly we adjusted. However, there is always a possibility of residual confounding, even after statistical adjustment (24,25). Moreover, the patients’ comorbidities and smoking/family history, that SEER has limited information could be a risk factor of secondary malignancies. For example, the high frequency of lung cancer also suggests the presence of tobacco-related comorbidity in RT patients. Pelvic radiation therapy is unlikely to increase the risk of lung cancer, and more smokers could explain the difference in secondary cancer incidence in the RT group. Also, we obtained limited information on adjuvant hormone therapy and chemotherapy, which are related to patients’ survival and their risks of secondary malignancy. Unfortunately, these data are not available and cannot be analyzed directly with the SEER database. Also, GS before the year 2004 was not available in the database, and we had a relatively short follow-up time (<10 years), which is not ideal for evaluating the incidence of secondary malignancies. The reason for this is that cancers could develop over a more extended period of time. Finally, in recent years, technological advances have decreased the morbidity caused by radiation.
Conclusions
In conclusion, men with clinically localized Pca should be better informed about the trade-off they have to make following any treatment, including cancer mortality, treatments outcomes, and potential adverse effects. Our study proved that the RT-induced risk of secondary malignancies is like the natural course of secondary malignancies development in young patients who have low-grade localized Pca. Although this risk appears to increase over time, the absolute rate remains small and therapeutic radiation in Pca survivors seems to be safe. Our study provides a useful addition to the existing evidence, which will help physicians and patients make decisions regarding their disease management. Longer-term follow-up and a higher level of evidence are essential to study this topic.
Table S1
| The entire cohort | No active treatment | RP | RT | P valuea | P valueb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Percentage (%) | N | Percentage (%) | N | Percentage (%) | N | Percentage (%) | ||||||
| Lung | 794 | 18.8 | 182 | 20.9 | 176 | 14.2 | 436 | 20.6 | 0.843 | <0.001 | |||
| Bladder | 520 | 12.3 | 95 | 10.9 | 123 | 9.9 | 302 | 14.3 | 0.015 | <0.001 | |||
| Colorectal | 465 | 11.0 | 103 | 11.8 | 126 | 10.2 | 236 | 11.1 | 0.612 | 0.388 | |||
| Melanoma | 370 | 8.8 | 69 | 7.9 | 168 | 13.5 | 133 | 6.3 | 0.109 | <0.001 | |||
| NHL | 245 | 5.8 | 48 | 5.5 | 79 | 6.4 | 118 | 5.6 | 1 | 0.362 | |||
| Total secondary malignancies | 4,228 | 100 | 870 | 100 | 1,241 | 100 | 2,117 | 100 | – | – | |||
a, P value was determined between RT and no active treatment cohort; b, P value was determined between RT and RP cohort. Pca, prostate cancer; RP, radical prostatectomy; RT, radiation therapy; NHL, non-Hodgkin lymphoma.
Table S2
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | 9 years | 9.9 years (119 months) | |
|---|---|---|---|---|---|---|---|---|---|---|
| No active treatment | 0.034% | 0.497% | 1.065% | 1.498% | 2.020% | 2.510% | 2.943% | 3.359% | 3.774% | 4.151% |
| RP | 0.020% | 0.262% | 0.537% | 0.801% | 1.140% | 1.418% | 1.754% | 2.044% | 2.406% | 2.774% |
| RT | 0.035% | 0.519% | 1.061% | 1.583% | 2.087% | 2.568% | 3.154% | 3.733% | 4.415% | 4.791% |
Pca, prostate cancer; RP, radical prostatectomy; RT, radiation therapy.
Table S3
| Unadjusted cohorta | Propensity-adjusted cohortb | ||||||
|---|---|---|---|---|---|---|---|
| N (Events) | HR (95% CI) | P value | N (Events) | HR (95% CI) | P value | ||
| Low risk group | |||||||
| RT vs. no active treatment (Ref.) | 18,451 [86] | 1.081 (0.706–1.657) | 0.720 | 8204 [39] | 1.546 (0.800–2.987) | 0.195 | |
| RT vs. RP (Ref.) | 11,821 [58] | 1.316 (0.647–2.679) | 0.448 | 817 [6] | 3.505 (0.411–29.905) | 0.252 | |
| Intermediate risk group | |||||||
| RT vs. no active treatment (Ref.) | 50,946 [1,312] | 1.047 (0.920–1.192) | 0.487 | 16,961 [505] | 0.959 (0.805–1.141) | 0.634 | |
| RT vs. RP (Ref.) | 120,630 [2,046] | 2.110 (1.935–2.301) | <0.001 | 29,235 [726] | 1.678 (1.450–1.942) | <0.001 | |
a, competing-risk analysis; b, Propensity-score matching and weighting with adjustment for age, year of diagnosis, marital status, race, PSA, Gleason score, AJCC T stage for competing-risk analysis. Pca, prostate cancer; RP, radical prostatectomy; RT, radiation therapy; HR, hazard ratio; CI, confidence interval; PSA: prostate-specific antigen; NCCN, The National Comprehensive Cancer Network.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure from (available at http://dx.doi.org/10.21037/tcr.2020.02.57). The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This analysis followed the Declaration of Helsinki and was exempt from ethics approval.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- American Cancer Society. Key statistics for prostate cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Berrington de Gonzalez A, Curtis RE, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 2010;102:220-6. [Crossref] [PubMed]
- Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med 1996;334:745-51. [Crossref] [PubMed]
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol 2014;32:2217-23. [Crossref] [PubMed]
- Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 2005;97:1354-65. [Crossref] [PubMed]
- Zelefsky MJ, Pei X, Teslova T, et al. Secondary cancers after intensity-modulated radiotherapy, brachytherapy and radical prostatectomy for the treatment of prostate cancer: incidence and cause-specific survival outcomes according to the initial treatment intervention. BJU Int 2012;110:1696-701. [Crossref] [PubMed]
- Murray L, Henry A, Hoskin P, et al. Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol 2014;110:213-28. [Crossref] [PubMed]
- Jin T, Song T, Deng S, et al. Radiation-induced secondary malignancy in prostate cancer: a systematic review and meta-analysis. Urol Int 2014;93:279-88. [Crossref] [PubMed]
- Wallis CJ, Mahar AL, Choo R, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ 2016;352:i851. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer. Version 2. 2018.
- Social Security Administration. Actuarial life table. Available online: http://www.ssa.gov/OACT/STATS/table4c6.html
- Preisser F, Bandini M, Mazzone E, et al. Validation of the Social Security Administration Life Tables (2004-2014) in Localized Prostate Cancer Patients within the Surveillance, Epidemiology, and End Results database. Eur Urol Focus 2019;5:807-14. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2016;375:1425-37. [Crossref] [PubMed]
- Barocas DA, Alvarez J, Resnick MJ, et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017;317:1126-40. [Crossref] [PubMed]
- Chen RC, Basak R, Meyer AM, et al. Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men With Localized Prostate Cancer. JAMA 2017;317:1141-50. [Crossref] [PubMed]
- Berrington de Gonzalez A, Wong J, Kleinerman R, et al. Risk of second cancers according to radiation therapy technique and modality in prostate cancer survivors. Int J Radiat Oncol Biol Phys 2015;91:295-302. [Crossref] [PubMed]
- Hegemann NS. Risk of second cancer following radiotherapy for prostate cancer: a population-based analysis. Radiat Oncol 2017;12:2. [Crossref] [PubMed]
- Warschkow R, Güller U, Cerny T, et al. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiother Oncol 2017;123:139-46. [Crossref] [PubMed]
- Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016;122:3075-86. [Crossref] [PubMed]
- Williams SB, Huo J, Chamie K, et al. Discerning the survival advantage among patients with prostate cancer who undergo radical prostatectomy or radiotherapy: The limitations of cancer registry data. Cancer 2017;123:1617-24. [Crossref] [PubMed]
- Roach M 3rd, Ceron Lizarraga TL, Lazar AA. Radical Prostatectomy Versus Radiation and Androgen Deprivation Therapy for Clinically Localized Prostate Cancer: How Good Is the Evidence? Int J Radiat Oncol Biol Phys 2015;93:1064-70. [Crossref] [PubMed]


