Prognostic value of Livin in surgical specimen and biopsy in patients with osteosarcoma
Introduction
OS, the most common malignant bone tumor, posing great impact on the health of children and adolescents (1,2). Although survival rate of OS patients was improved after the improvement of combined chemotherapy and surgery, fatal metastasis was still a significant factor in reducing survival rate of OS patients (3). Currently, the underlying mechanism of OS are still not completely clarified. Furthermore, there is no effective method to monitor OS metastases early. Therefore, it is imperative to find the robust prognostic biomarkers of metastatic OS (4,5). Active intervention and treatment can be applied to those patients with a higher risk after initial diagnosis to improve their prognosis, according to high expression of certain biomarkers. So far, positive expression of Livin has been considered as a OS prognosis-related biomarkers (6).
Many studies have recently revealed that the development of OS is strongly linked to apoptosis of cell (7-9), and the over-expression of apoptosis-related protein Livin is strongly correlated with the malignancy level of many epithelial cell carcinoma (6). Tumors with high expression of Livin are characterized by strong migration and high invasiveness. These tumors have poor sensitivity to chemotherapy drugs with poor prognosis. Apoptosis-related protein Livin was a newly recognized member of inhibition apoptosis protein (IAP) discovered in recent years. The human IAP family was comprised of 8 members, and Livin was an essential member of IAP family. Its gene is located on human chromosome 20q13.3 with full length of 4.6 kb, containing 6 introns and 7 exons, and it contains a single baculovirus IAP repeat domain and a carboxy terminal structure (6), which is an important molecule with anti-apoptotic effects. The anti-apoptotic mechanisms mainly through caspases-dependent and non-caspases-dependent pathways. Livin can bind to caspases-3 and caspases-7 to inhibit its activity (10). In addition, When Livin binds to JenyTAB1, it can activate TAK1, which in turn activates JNK1 and then induces anti-apoptotic effects via the TAK1/JNK1 pathway. Livin wasn’t expressed in tissues of healthy adults. On the contrary, it is specifically expressed in certain solid tumor tissues, such as, gastrointestinal tumors and even most melanoma cell lines. The research data showed that Livin was highly expressed in OS tissues. Livin was not only involved in tumor proliferation and apoptosis, but also closely related to tumor cell invasion, metastasis and sensitivity to chemotherapeutic drugs. To investigate the relationship between Livin and metastasis as well as survival, here we performed this meta-analysis of reports about association between Livin positive expression and OS.
Methods
Search methods and report selection
A systematic search using CBM, Chinese VIP database, Wanfang database, CNKI, Springer, ISI Web of Knowledge, the Cochrane library, Embase and NCBI PubMed was conducted to further explore the clinical relevance of Livin positive expression and prognosis of OS. The last search was conducted on November 17, 2018. The search was executed by two investigators and a combination the keyword terms: (Livin) and (OS, osteosarcoma or osteogenic tumor) were used in the search methods for each database.
Exclusion and inclusion criteria
Exclusion studies: (I) editorials, letters, expert opinions, talks, correspondences, case reports, cell or animal experiments and reviews with no original research data; (II) reports with non-dichotomous Livin expression levels; (III) paper without cut-off value; (IV) the same author’s similar studies; (V) earlier and smaller sample data excluded for many duplicate data; (VI) studies without survival outcome were excluded; (VII) OS was not confirmed by biopsy.
Inclusion studies: (I) papers with confirmation of OS in patients based on pathological diagnosis (gold standard); (II) reports indicating Livin positive expression measured with commercial reagents; (III) the research must provide enough information to construct 2×2 contingency table; (IV) papers published in Chinese or English.
Data extraction
Two investigators assessed whether all retrieved studies were qualified and independently extract relevant data. And then they examined each other's extracted databases to rule out any differences. We extracted the information included first authors’ name, published date, methods of Livin measurement and the cut-off definition of Livin positive expression. The corresponding authors of each trial were contacted if the information we need was not mentioned on published papers. The study was excluded in case further information is not available to ensure the accuracy of the results.
Evaluation of included publications
We performed quality assessment on each included study using NOS as described in our previous study (1).
Statistical analysis
The statistical analysis was conducted as described in our previous study (1).
Ethics statement
Not applicable.
Results
Eligible studies
Forty-seven potentially relevant papers were retrieved from our initial literature search. After deleting all unqualified records, review papers and items lacking necessary information by manual check, 10 studies comprised of 439 patients were retained for our qualitative research (7,11-19). The detailed screening process is shown in Figure 1. Finally, there are 27 to 64 patients with OS in each included publications (median: 45.5). The characteristics of enrolled studies for OS metastasis and survival were summarized in Tables 1 and 2 respectively.
Table 1
| Ref | Study | Year | No. of patients | Age (mean or median) | Method | Assay kit | Livin cut-off | Livin positive | Livin negative | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metastasis | Non metastasis | Metastasis | Non metastasis | ||||||||||
| (16) | An et al. | 2008 | 45 | 36.5 | IHC | SABC | A2×B2 ≥1 | 15 | 13 | 1 | 16 | 8 | |
| (7) | Liu et al. | 2008 | 45 | 14.5 | IHC | Imgenex | A1 ≥2 | 17 | 11 | 2 | 15 | 8 | |
| (18) | An et al. | 2007 | 45 | 21.8 | IHC | SABC | A2×B2 ≥1 | 15 | 13 | 1 | 16 | 7 | |
| (15) | Xie et al. | 2010 | 27 | 10.6 | IHC | OriGene | A1 ≥2 | 12 | 4 | 5 | 6 | 8 | |
| (14) | Zhang et al. | 2015 | 58 | 19.4 | IHC | Sangon | A1×B1 ≥3 | 36 | 4 | 12 | 6 | 7 | |
A: positive cell percentage—A1: scored 1 (<25%), 2 (26–50%), 3 (51–75%), 4 (>75%); A2: scored 0 (<5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), 4 (>75%). B: staining intensity—B1: scored 0 (absence of staining), 1 (weak staining), 2 (strong staining); B2: scored 1 (weak staining), 2 (moderate staining), 3 (strong staining).
Table 2
| Ref | Study | Year | No. of patients | Age (mean or median) | Method | Assay kit | Livin cut-off | Livin positive | Livin negative | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death | ≥3-year survival | Death | ≥3-year survival | ||||||||||
| (11) | Fu et al. | 2016 | 64 | 19.8 | IHC | MaxVisionTM | A1×B1 ≥3 | 37 | 6 | 4 | 17 | 7 | |
| (17) | Sun et al. | 2016 | 48 | 22.6 | IHC | Abnova | A2+B2 >2 | 14 | 14 | 4 | 16 | 8 | |
| (12) | Ji et al. | 2015 | 39 | 20.5 | IHC | Max Vision | A3+B3 >2 | 17 | 6 | 6 | 10 | 7 | |
| (14) | Zhang et al. | 2015 | 58 | 19.4 | IHC | MaxVisionTM | A4+B4 ≥3 | 20 | 15 | 7 | 16 | 7 | |
| (13) | Li et al. | 2014 | 39 | 25.5 | IHC | Max VisionTM/AP | A5+B3 >2 | 17 | 6 | 6 | 10 | 8 | |
| (19) | Nedelcu et al. | 2007 | 29 | 22.3 | IHC | Alpha | A1 >2 | 3 | 0 | 7 | 19 | 7 | |
A: positive cell percentage—A1: scored 1 (<10%), 2 (10–50%), 3 (>50–75%), 4 (>75%); A2: scored 0 (=0), 1 (1–25%), 2 (26–50%), 3 (> 50%); A3: scored 0 (<5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), 4 (>75%); A4: scored 1 (1–25%), 2 (26–50%), 3 (51–75%), 4 (75–100%); A5: scored 0 (<5%), 1 (6–35%), 2 (36–70%), 3 (>70%). B: staining intensity—B1: scored 1 (pale yellow), 2 (brownish yellow), 3 (tan); B2: scored 0 (absence of staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining); B3: scored 0 (absence of staining), 1 (pale yellow), 2 (Brownish yellow), 3 (tan); B4: scored 0 (absence of staining), 1 (weakly positive), 2 (strongly positive).
Qualitative evaluation
In the present study, we assessed the quality of enrolled reports by NOS. The quality scores of included papers ranged from seven to eight points (median: 7.42). The quality assessment scores were indicated in Tables 1,S1.
Meta-analysis
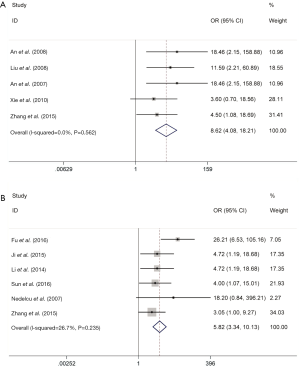
I2 test was used to assess the heterogeneity of enrolled publications. I2 values close to zero represent homogeneity and low heterogeneity was identified as I2 ≤50%. Significant heterogeneity was identified if I2 >50%. In a meta-analysis to evaluate the relationship of Livin positive expression and OS prognosis, the result showed no significant heterogeneity (I2 ≤50%). Therefore, fixed effect model was adopted to calculate the combined OR and 95% CI. As pooled OR for all enrolled publications indicating, metastasis was more likely to occur in OS patients with Livin positive expression (OR =8.62, 95% CI: 4.08–18.21, P<0.0001), and it was logical that these patients tend to have lower 3-year survival rates (OR =5.82, 95% CI: 3.34–10.13, P<0.001). In total, Livin positive expression presented higher risk of metastasis and lower 3-year survival rates of OS patients (Figure 2).
Sensitivity analysis
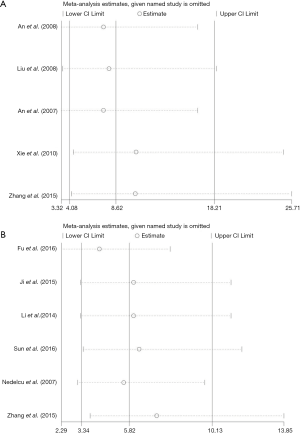
We conducted a sensitivity analysis to evaluate the stability of obtained results, showing that combination of the OR was stable, and the heterogeneity didn’t vary significantly when a single study was removed. We assessed the robustness of the results by eliminating one study at a time and recalculating the overall OR. A one-time sensitivity analysis was performed to indicate that our analysis was less dependent on the study and that the conclusions were stable (Figure 3). These data suggested that Livin positive expression might be a reliable prognostic factor in OS patients.
Publication bias
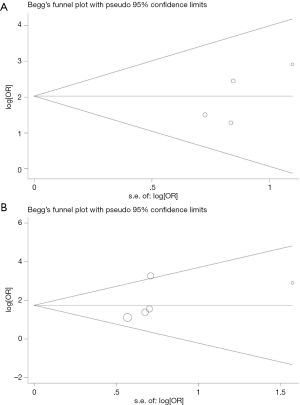
In order to detect publication bias of studies included in the present study, we used Begg funnel plot. According to the result, the funnel plot had no obvious evidence of asymmetry among 10 papers (Figure 4). In addition, Egger test also showed no significant publication bias in this meta-analysis (P>0.05).
Discussion
OS, a common life-threatening primary malignant bone tumor among adolescents (20), is characterized by poor prognosis and insensitivity to chemotherapy and radiotherapy. The disease-free survival rate improved from <20% to about 60% with the introduction of effective chemotherapy (21). However, this situation is not satisfactory for the tumor are prone to relapse and metastasis. Because of low sensitivity to chemotherapy and radiotherapy, the OS prognosis remains poor. Although five-year survival rate of patients with OS has increased significantly over the past few decades, metastasis, especially in the lungs, is still the leading cause of OS patient’s death (22). The treatment of OS is currently at a bottleneck stage. OS is easy to metastasize relapse and early metastasis of OS is a major factor affecting if a patient's cure rate can be improved. So that, it is essential to investigate novel prognostic biomarkers to identify at-risk patients and aid clinical decision-making. At present, it is well-documented that positive expression of biomarkers indicates the poor prognosis of OS. Livin is one of the potential prognostic indicators of OS. The reduction of anti-apoptotic factors may provide a reasonable basis for the development of new strategic targets for the treatment of OS (23). IAPs are the only known endogenous proteins that regulate the activity of promoters and effector caspases (24). Livin, a novel member of IAP family, was found in many types of cancer, including breast and prostate cancer, OS, melanoma, and lymphoma cells (24). According to many studies, Livin antagonizes death receptor and mitochondrial apoptosis signaling pathways by inhibiting caspase-3 and other caspase enzymes, leading to inactivation and degradation (25). Livin with the anti-apoptotic potential indicated that the regulation of Livin expression can be used as a new target for tumor therapy (26). Previous study found that the high expression of Livin in tumor tissues was positively correlated with chemotherapy resistance of tumors (27). Therefore, selective Livin inhibitors have potential applications in preventing OS. These papers indicated that Livin play a significant role in tumor metastasis and invasion. Livin may be a potential biomarker indicating prognosis of OS.
Meta-analysis is a quantitative method that combines information from different research-related topics to facilitate the evaluation of cancer-related prognostic indicators (28). In order to accurately assess the value of prognosis of Livin-positive expression in OS, here we conducted a meta-analysis with 10 published papers included. The results showed that the positive expression of Livin in OS predicted that Livin was statistically significant in OS metastasis (OR =8.62, 95% CI: 4.08–18.21, P<0.0001) and OS survival (OR =5.82, 95% CI: 3.34–10.13, P<0.001). In addition, we performed a sensitivity analysis to detect the stability of obtained results. The pooled OR was stable and did not change significantly after removing a single study. This meta-analysis showed that Livin was a potential biomarker for guiding OS clinical treatment.
Nonetheless, our finding is preliminary and there are certain limitations to our study that have to be addressed. First, there is no publication bias in these included studies, but manuscripts with expected results are more likely to be published, which can contribute to bias in overall accuracy. Second, articles included in this study were only published in English or Chinese, which is likely to impact on the results. Third, the total sample size involved in this study was small with an average of 44. In addition, 255 OS patients were positive for Livin and only 184 patients were negative for Livin. Due to the relatively small size, random errors and sample deviations are inevitably generated.
Conclusions
In summary, we synthesized the related research by means of a systematic review and meta-analyses to assess the relationship of positive expression of Livin and OS prognosis. The obtained results showed that Livin was an effective biomarker and have the potential for clinical application. Livin positive expression was significantly related to increased risk of metastasis and worse survival outcomes in patients with OS. However, in order to evaluate the prognostic value of Livin-positive expression, there is still a need for more elaborate studies with larger sample sizes.
Table S1
| Column | Entries | Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Section | Is the definition adequate | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Representativeness of the cases | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Selection of controls | |||||||||||
| Definition of controls | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Comparability | Comparability of cases and controls on the basis of the design and analysis | ☆☆ | ☆☆ | ☆ | ☆☆ | ☆ | ☆ | ☆☆ | ☆ | ☆☆ | ☆ |
| Exposure | Ascertainment of exposure | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Same method of ascertainment for cases and controls | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Non-response rate | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Total scores | 8 | 8 | 7 | 8 | 7 | 7 | 8 | 7 | 8 | 7 | |
1: An
Acknowledgments
We would like to thank Dr. Ayub Abdulle nur and Dr. Lifang Wen for English language support in preparing revised manuscript.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1979). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou J, Wang W, Yan Q, et al. Expression of HER-2 in surgical specimen and biopsy as a biomarker of metastasis in patients with osteosarcoma: a meta-analysis. Transl Cancer Res 2019;8:1129-36. [Crossref]
- Ritter J, Bielack SS. Osteosarcoma. Ann Oncol 2010;21:vii320-5. [Crossref] [PubMed]
- Bielack SS, Hecker-Nolting S, Blattmann C, et al. Advances in the management of osteosarcoma. F1000Res 2016;5:2767. [Crossref] [PubMed]
- Zhou J, Xiao X, Wang W, et al. Association between PTEN and clinical-pathological features of osteosarcoma. Biosci Rep 2019;39. [PubMed]
- Xu N, Kang Y, Wang W, et al. The prognostic role of CD133 expression in patients with osteosarcoma. Clin Exp Med 2020;20:261-7. [Crossref] [PubMed]
- Li X, Fan S, Li L, et al. RNA interference-mediated knockdown of Livin suppresses cell proliferation and invasion and enhances the chemosensitivity to cisplatin in human osteosarcoma cells. Int J Oncol 2013;43:159-68. [Crossref] [PubMed]
- Liu J, Sun Y, Meng Q, et al. Expression of Livin and Fas in osteo-sarcoma. Progress Mod Biomed 2008;8:1465-7.
- Li J, Yang Z, Li Y, et al. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget 2016;7:44763-78. [Crossref] [PubMed]
- Yong L, Ma Y, Zhu B, et al. Oleandrin synergizes with cisplatin in human osteosarcoma cells by enhancing cell apoptosis through activation of the p38 MAPK signaling pathway. Cancer Chemother Pharmacol 2018;82:1009-20. [Crossref] [PubMed]
- Xu M, Xia LP, Fan LJ, et al. Livin and caspase-3 expression are negatively correlated in cervical squamous cell cancer. Eur J Gynaecol Oncol 2013;34:152-5. [PubMed]
- Fu W, Chang N, Wang S, et al. Expression and clinical significance of Livin and Caspase-8 in tissues of human osteosarcoma. CHINA MEDICAL HERALD 2016;13:33-6.
- Ji Z, Li H, Zhong Z, et al. Expression and clinical significance of Livin and MAGE-A1 in human osteosarcoma. Chin J Hypertens 2015;23:339-41.
- Li H, Sun K, Cai F, et al. Expression and clinical significance of Livin and Caspase-7 protein in human osteosarcoma tissues. Chin J Cancer Biother 2014;21:320-4.
- Zhang L. Correlation between the expression of Livin and VEGF in osteosarcoma and patient's prognosis. Hebei Medical Journal 2015;37:1944-7.
- Xie C, Wu B, Zhao Y, et al. Association between Livin expression and microvascular density in osteosarcoma. The Journal of Practical Medicine 2011;27:1529-32.
- An G, Lv G, Zeng Y, et al. Expressions of Livin and Caspase-3 in human osteosarcoma and their clinical significance. Chin J Cancer Prev Treat 2008;15:1734-6.
- Liu J, Sun Q, Sun Y, et al. Livin and Caspase-3 expression in osteosarcoma. Chin J Bone Tumor Bone Disease 2009;8:32-4.
- An G, Lv G, Tu G, et al. Expression and significance of apoptosis inhibitor Livin in osteosarcoma. Shandong Medical Journal 2007;47:66-7.
- Nedelcu T, Kubista B, Koller A, et al. Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res Clin Oncol 2008;134:237-44. [Crossref] [PubMed]
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol 2010;8:705-18. [PubMed]
- Osborne TS, Khanna C. A review of the association between osteosarcoma metastasis and protein translation. J Comp Pathol 2012;146:132-42. [Crossref] [PubMed]
- Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res 2009;152:239-62. [Crossref] [PubMed]
- Horie R, Nakamura O, Yamagami Y, et al. Apoptosis and antitumor effects induced by the combination of an mTOR inhibitor and an autophagy inhibitor in human osteosarcoma MG63 cells. Int J Oncol 2016;48:37-44. [Crossref] [PubMed]
- Saleem M, Qadir MI, Perveen N, et al. Inhibitors of apoptotic proteins: new targets for anticancer therapy. Chem Biol Drug Des 2013;82:243-51. [Crossref] [PubMed]
- Liu GH, Wang C, Ding ZY. Overexpression of the truncated form of Livin reveals a complex interaction with caspase-3. Int J Oncol 2013;42:2037-45. [Crossref] [PubMed]
- Lin X, Li HR, Lin XF, et al. Silencing of Livin inhibits tumorigenesis and metastasis via VEGF and MMPs pathway in lung cancer. Int J Oncol 2015;47:657-67. [Crossref] [PubMed]
- Wang X, Xu J, Ju S, et al. Livin gene plays a role in drug resistance of colon cancer cells. Clin Biochem 2010;43:655-60. [Crossref] [PubMed]
- Xiao D, Wang Y, Xu B. Introduction and Inspiration of Meta-Analysis. Medicine & Philosophy 1998;19:179-82.