Significance of genetic sequencing in patients with lung adenocarcinoma with transformation to small cell lung cancer: a case report and systematic review
Introduction
Lung cancer is the leading cause of cancer-related death. Based on its biological characteristics, lung cancer is divided into two categories: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Adenocarcinoma is currently the most common histological subtype. Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) exhibit beneficial therapeutic effects on adenocarcinoma with the EGFR sensitive gene mutation. The National Comprehensive Cancer Network (NCCN) identified EGFR-TKI as a first-line treatment for patients with advanced EGFR-mutated lung adenocarcinoma (1). However, disease progression inevitably occurs after a period of ~12 months of treatment (2,3). A variety of mechanisms are associated with the development of the acquired resistance to EGFR-TKIs, including the T790M mutation, c-MET amplification, KRAS mutation, BIM polymorphism deletion and PIK3CA gene mutations. One of them was surprising-NSCLC transformation into SCLC, 3–15% of NSCLC cases transform into SCLC (4-6). Historically, SCLC was believed to develop from neuroendocrine cells of central airways, whereas adenocarcinoma derives from the alveolar type II cells located in the alveolar surface area. Several studies have shown that alveolar type II cells may be common precursors of both lung adenocarcinoma and SCLC (5,7). The transformation from adenocarcinoma to a SCLC phenotype was first reported by Zakowski in 2006 (8). The mechanisms underlying the NSCLC-to-SCLC transformation after TKI therapy still remains unclear. There were also some case reports about SCLC transformation from EGFR-mutated lung adenocarcinoma (9). But generally, SCLC transformation is only found by the change of tumor markers or pathological examination. Different from previous case reports, pathological examination and genome sequencing were carried out when every time the disease progressed, we obtained more comprehensive information and could keep track of the patient's progress. So, we could adjust the treatment plan at any time according to the results of pathological examination and gene detection. Hence, we report a case of TKI resistance due to SCLC transformation and review the literature to summarize the clinical features, mechanisms, predictors of SCLC transformation, treatment after transformation. One case of a 56-year-old man with lung adenocarcinoma is reported, who exhibited an EGFR mutation (19-Del) and was treated with EGFR-TKIs. This case transformed into SCLC after failed to EGFR-TKI targeted therapy. The patient received chemotherapy, EGFR-TKI, vascular-targeted therapy and immunotherapy. Through the analysis of diagnosis and treatment, the relevant mechanisms and treatment strategies are summarized in the current paper. This report suggested that combination treatment using cytotoxic chemotherapy plus an EGFR-TKI may be useful for patients with EGFR-mutated adenocarcinoma and transformed small cell carcinoma. If the genetic testing done, maybe we can screen out the high-risk population of small cell transformation. We present the following case in accordance with the CARE Guideline.
Case presentation
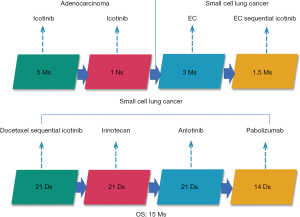
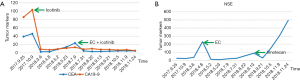
A 56-year-old Asian man with a 25-year history of smoking 40 cigarettes per day was presented to the outpatient clinic with symptoms of a cough and expectoration that had lasted 1 month since Sep 2017. There was no family history of carcinoma and general health was good. On examination: dullness to percussion at the bases and auscultation revealed respiratory sounds in the left lower lobe were weak; the rest were normal. A positron emission tomography/computed tomography (PET-CT) scan indicated: a mass shadow in the left upper lobe of the lung, multiple metastases in the lymph nodes of the mediastinal, multiple metastasis in the left pleural and a large accumulation of fluid in the left pleural. Histopathology and immunohistochemistry results revealed adenocarcinoma. The patient was diagnosed with stage IVa (cT3N3M1a) according to the eighth edition of the clinical tumor-node-metastasis staging system. Genome sequencing indicated the presence of exon 19 deletion mutations (57.30%), EGFR copy number amplification (CN =4.7), RB1 and TP53 mutations. The patient had received treatment with icotinib (125 mg, three times a day) since November 2017, within the first 2 weeks of therapy the cough and expectoration resolved and the patient’s performance status improved and stayed stable. And he exhibited a partial response (PR) 3 months after treatment initiation [carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) decreased to normal levels]. A CT scan performed in March 2018 revealed a trend in progression of the disease (PD). Neuron specific enolase (NSE) were also indicated to be increased significantly. A pathological examination (US-guided needle aspiration biopsy in pleural cavity tumor) revealed compound small cell carcinoma in April 2018 (Figure 1). Genome sequencing revealed exon 19 mutations (67.58%), EGFR copy number amplification, RB1 and TP53 mutations, missense mutations in exon 4 of MSH6, PMS2 copy number amplification and no T790M mutations; PD-L1(–), microsatellite stable (MSS), tumor mutational burden (TMB) 15.32 Muts/Mb (Table 1). From April 2018 the patient was treated using carboplatin and etoposide (EC). The patient exhibited a PR after a two-cycle treatment (NSE decreased to normal levels), but PD after four treatment cycles. From June 2018 the patient was treated using carboplatin and etoposide sequential icotinib (CEA was higher) (Figure 2). After a subsequent two cycles, the disease progressed further (Figure 3). The patient was then treated with docetaxel sequential icotinib, irinotecan, anlotinib and pabolizumab, and after one cycle, the illness has advanced further. Adverse effects were seen in the patient when treated with pabolizumab, included pancreatitis, thyroiditis, myocarditis. The major adverse reactions were mild. All these symptoms resolved completely after discontinuation of pabolizumab and symptomatic treatment. However, malignancy tumor progress was rapid, the patient died in January 2019 and had an overall survival (OS) of 15 months (Figure 4).
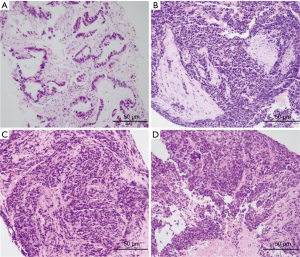
Table 1
| Gene name | 2017.10.17 | 2018.3 | 2018.4.18 | 2018.4.22 | 2018.7.21 |
|---|---|---|---|---|---|
| EGFR exon 19 deletion mutations | 57.3% | 67.58% | Y | 49.93% | 61.51% |
| EGFR copy number amplification (CN) | 4.7 | 4.68 | – | 3.14 | 3.69 |
| TP53 non-shift deletion mutation in exon 5 | 34.05 | 76.73 | Y | 34.47 | 55.94 |
| RB1 frameshift mutation in exon 2 | 48.09 | 84.03 | – | 44.79 | 70.26 |
| MSH2 | – | – | Y | – | – |
| MSH6 missense mutation in exon 4 p. D904E | – | 26.59 | Y | – | – |
| PMS2 copy number amplification (CN) | – | 4.96 | – | 3.26 | 3.89 |
| TMB (Muts/Mb) | – | ≤10 | 15.32 | ≤10 | 9.5 |
Y, the result was positive; –, the result was negative. EGFR, epidermal growth factor receptor; CN, copy number; TMB, tumor mutational burden.

Discussion
The mechanisms of the transformation from NSCLC to SCLC after the failure of EGFR-TKI therapy
SCLC accounts for ~15% of lung cancer cases (10) and is an aggressive disease characterized by rapid growth and early widespread metastasis. Metachronous SCLC has also been diagnosed in repeated biopsies from NSCLC patients who had previously received cytotoxic chemotherapy (4,6,8,11-14). However, SCLC containing the EGFR mutation is transformed from lung adenocarcinoma, or is combined with small cell lung cancer (CSCLC) at first diagnosis. The incidence of the SCLC phenotype is controversial. It has been previously reported that the EGFR gene mutation rate in patients with SCLC is low (~4%) (15), and EGFR-TKIs are not effective (16). Some studies have investigated the transformation mechanisms of SCLC throughout the course of treatment, from EGFR-TKIs treatment to SCLC transformation. Multigene detection was performed in four patients with tumors at multiple time points. The results revealed that EGFR mutant adenocarcinoma and SCLC originated from a common primitive clone, and this branching occurred prior to the initial treatment with EGFR-TKIs. EGFR-TKI resistant mutations were only revealed in lung adenocarcinoma clusters (these include the T790M mutation and EGFR amplification). Classical variants of SCLC (including the PIK3CA mutation and MYC amplification) were only revealed in SCLC clusters. However, a study using small sample sizes does not exclude the possibility of linear evolution. It is therefore necessary to perform prospective studies using larger sample sizes to further understand the mechanisms of SCLC transformation. The aforementioned study also demonstrated that double mutations of TP53 and RB1 in patients with lung adenocarcinoma and the EGFR mutation predicted SCLC transformation. The rate in the SCLC transformation group was higher than that in the non-transformation group (82% vs. 3%) and the risk of SCLC transformation was 42.8 times higher in patients with TP53 and RB1 mutations (17). TP53 and RB1 double mutations are the most common gene mutations in SCLC, occupying 32% of all types of gene mutation (18), but these mutations are rare in common lung adenocarcinoma (19). The analysis of NSCLC specimens and cell lines which exhibited resistance to EGFR-TKI revealed that the incidence of RB gene inactivation in NSCLC-transformed SCLC is almost 100% and is therefore a necessary condition for the development of SCLC. However, RB gene inactivation is rare in NSCLC with EGFR-TKI resistance but without SCLC transformation (20). Puncture biopsy is recommended in patients with lung adenocarcinoma and EGFR-TKI mutations and TP53 and RB1 double mutations when EGFR-TKI treatment fails, instead of relying on liquid biopsies. Detection plasma may be helpful to determine whether a T790M mutation is present, however, histological transformation will not be detected (21). In this patient, TP53 and RB1 double mutations were detected using polygene sequencing at initial diagnosis. SCLC transformation occurred 5 months after EGFR-TKI treatment. Roca et al. have found that the median time from initial diagnosis of lung adenocarcinoma to the transformation to SCLC was 19 (range, 1–61) months. There is great uncertainty in the time span (22). NSE and a variety of other SCLC-related tumor markers exhibited normal values prior to and during treatment with EGFR-TKI. NSE increased significantly after secondary resistance of the targeted treatment and the pleural tumor subsequently enlarged rapidly. NSE decreased rapidly after EC regimen chemotherapy and the pleural tumor was reduced and nearly disappeared. This occurred in accordance with the biological characteristics of SCLC. The possibility of transformation to SCLC should be considered when treating EGFR-mutant adenocarcinoma with EGFR-TKI, especially when serum NSE increases. And the one that should cause attention even more is the patients with TP53 and RB1 mutations.
Therapy-respond guide treatment strategy
In a clinical setting, how transformation of SCLC can be predicted or detected as early as possible deserves increased attention. There may be an increased risk of SCLC transformation in NSCLC with neuroendocrine differentiation (expressing NSE) or with RB and/or TP53 mutations. When EGFR-TKI treatment fails, due to the accumulation of resistant mutations, SCLC transformation should be considered. After SCLC transformation, therapeutic strategies should be adjusted. SCLC is highly malignant and develops rapidly. Therefore, SCLC treatment should be a priority (the most commonly used regimen is etoposide plus platinum). It has been revealed that not all tumors transform into SCLC and some partial lesions remain as adenocarcinoma (17,23). Consideration should, however, be given to the original composition of adenocarcinoma after SCLC is controlled. The CEA of this patient increased slowly during EC treatment. Icotinib was added after the fifth cycle of carboplatin and etoposide and a reduction in CEA was subsequently observed. But, overall, the prognosis after SCLC diagnosis is poor and current treatment strategies derived from primary SCLC seem to be largely inefficacious.
Immunotherapy
In recent years, immunotherapy has demonstrated good antitumor activity, particularly when using programmed death receptor-1/ligand-L1 (PD-1/L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4). SCLC has a high immunogenicity (24) and mutation load (25), among other favorable immune factors, meaning that immuno-checkpoint inhibitors may become important for the treatment of SCLC. To assess the relationship between TMB and objective response rate (ORR) in 27 types or subtypes of tumors treated with PD-1/L1 inhibitors, studies have summarized ORR data from various large-scale studies and revealed that TMB is highly correlated with ORR in multiple tumor treatment using PD-1/L1 inhibitors (26). CheckMate032 research demonstrated a decreased prevalence of PD-L1 expression in SCLC, with only 17% of cases exhibiting >1% expression (27). Furthermore, SCLC efficacy did not increase with increased PD-L1 expression. In the high TMB sub-group: ORR, mPFS and mOS in the nivolumab and nivolumab + ipilimumab group (21.3% vs. 46.2%), (1.4 vs. 7.8 months), (5.4 vs. 22 months); in the low or medium TMB sub-group: ORR, mPFS and mOS in the nivolumab and nivolumab + ipilimumab group (4.8% vs. 22.2% or 6.8% vs. 16%), (1.3 vs. 1.5 months or 1.3 vs. 1.3 month), (3.1 vs. 3.4 months or 3.9 vs. 3.6 months) (28). In patients with high TMB, ORR, mPFS and mOS were significantly improved in the combination group and for patients with low or moderate TMB, ORR was improved, but mPFS and mOS were not improved. Some studies have revealed that TMB in patients with EGFR mutant lung adenocarcinoma was lower than that in patients with wild-type lung adenocarcinoma and NSCLC patients with TP53 or KRAS mutations. TMB has been indicated to significantly increase in patients with TP53 and KRAS mutations and this may mean they are more sensitive to PD-1/PD-L1 drugs (29). These results indicate that TMB may be a potential biomarker for predicting the efficacy of immunotherapy. However, TMB reflects the mutational landscape and therefore requires additional technical study and the cutoff values for scoring TMB remain disputed.
Mismatch repair (MMR) is an important DNA repair mechanism. During DNA replication and recombination, MMR mechanisms can identify and repair the mismatch, deletion and insertion of base pairs. MMR protein deficiencies are caused by mutations in MMR-associated genes including MLH1, MSH2, MSH6 and PMS2 and is reffered to as MMR gene defect (MMR deficient, dMMR) (30). dMMR results in the accumulation of somatic mutations and the appearance of multiple alleles at microsatellite loci. This is known as microsatellite instability (MSI). Previous studies have suggested that dMMR/MSI exhibit a positive correlation with high TMB (31), that dMMR/MSI produce large quantities of new antigens and improve the efficacy of PD-1 antibody A prospective clinical study assessed the efficacy of PD-1 antibody (pembrolizumab) in 12 different advanced cancer types with dMMR. In the aforementioned study, a total of 86 patients were enrolled. The disease was controlled in 77% patients, 53% patients had an objective imaging response and 21% patients achieved complete remission. The median follow-up time was 12.5 months and mPFS and mOS were not achieved (32). In 2017, pembrolizumab was approved for the treatment of patients with dMMR/MSI-high regardless of cancer type, indicating that dMMR/MSI-high is a universal genetic alteration in tumors.
The patient case assessed in the current paper exhibited a TMB of 15.32 Muts/Mb and deficiency in DNA MMR genes MSH6, MSH2 and PMS2. If dMMR causes a loss of MMR deficiency at the protein level, immunotherapy could be a beneficial treatment for this patient. But the presence of PD-L1(–) and MSS suggest that the effect of immunotherapy may be poor. This patient was subsequently treated with pembrolizumab and immunotherapy proved to be ineffective in this patient. Due to the high heterogeneity of tumors, it is difficult to accurately calculate TMB. More comprehensive and accurate predictors of immune marker expression are required to subsequently select patients who may benefit from immunotherapy. Further study is required to establish if patients with transformed SCLC can benefit from immunotherapy.
This case provides valuable information for clinical practice. As the disease becomes increasingly advanced, the heterogeneity of tumors becomes more complicated. Varying organs, time periods and discrepancies between organ histomorphology and molecular characteristics are present. Therefore, single-site and single-point detection is insufficient. Disease assessments should be undertaken in multiple parts and as a dynamic inspection. Additionally, comprehensive genetic testing is increasingly important. Patients with EGFR mutant lung adenocarcinoma with the double inactivation of RB1 and TP53 genes exhibit an increased risk of SCLC transformation. It is therefore recommended that puncture biopsy be performed instead of liquid biopsy for SCLC diagnosis. After SCLC transformation, therapeutic strategies should be adequately adjusted. SCLC is highly malignant and develops rapidly, so the treatment of SCLC should be a priority and consideration should still be given to the original composition of adenocarcinoma after SCLC is controlled. The treatment can be adequately adjusted when SCLC transformation is predicted or detected as early as possible. This also avoids the potential missed opportunities for further treatment due to the rapid development of SCLC. Evidence of the benefits of immunotherapy in patients with SCLC transformation is insufficient. However, immunotherapy is being increasingly promoted as a SCLC treatment option.
The achievement of the SCLC phenotype is a late phenomenon during TKI therapy and the prognosis of patients after SCLC diagnosis is poor. Current treatment strategies, adopted in the management of primary SCLC, appear to be largely inefficacious in transformed SCLC, and novel treatment approaches are needed. The mechanisms underlying the NSCLC-to-SCLC transformation after TKI therapy still remain undetermined and predictive markers are required. Phenotypic transformation and EGFR mutation have been associated with this transformation, however, cell heterogeneity, cancer stem cells or other associated molecular events (TP53 and RB1) may be involved. A major limitation of the present study is that patient data were retrospectively collected from published articles. Therefore, the number of cases from which to draw observations from was low. The second biopsy was taken from the same anatomical location as the first and the timing of specimen collection was also inconsistent. Therefore, the therapeutic recommendations of the current study are limited to a small sample and provide a low level of evidence for use in clinical practice. Collecting a large number of cases for a comprehensive prospective study (including biological characteristics, response rate to current treatment measures and prognosis) is an issue that requires future attention.
But our case has some limitations. Firstly, the biopsy method was fine needle aspiration and biopsy under the guidance of color Doppler, the cancer biopsies was relatively few. Secondly, the patient had not carried out genetic detecting since receiving docetaxel treatment, we did not know if the gene state changed again. And this was just a retrospective case report, more accurate research and treatment information can be assessed to provide evidence-based medical recommendations.
Acknowledgments
We owe thanks to the patient and his family. We thank the staff at Tianjin Medical University Cancer Institute and Hospital.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2291). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board at the Tianjin Medical University Cancer Institute and Hospital. Written informed consent has been provided by the patient’s next-of-kin to have the case details and any accompanying images published. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin 2015;25:185-97. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008;14:2895-9. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [Crossref] [PubMed]
- Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013;8:1265-71. [Crossref] [PubMed]
- Shi X, Duan H, Liu X, et al. Genetic alterations and protein expression in combined small cell lung cancers and small cell lung cancers arising from lung adenocarcinomas after therapy with tyrosine kinase inhibitors. Oncotarget 2016;7:34240-9. [Crossref] [PubMed]
- Zakowski MF, Ladanyi M, Kris MG, et al. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med 2006;355:213-5. [Crossref] [PubMed]
- Liu Y. Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: a case report and literatures review. Cancer Biol Ther 2018;19:445-9. [Crossref] [PubMed]
- Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev 2015;29:1447-62. [Crossref] [PubMed]
- Popat S, Wotherspoon A, Nutting CM, et al. Transformation to "high grade" neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;80:1-4. [Crossref] [PubMed]
- van Riel S, Thunnissen E, Heideman D, et al. A patient with simultaneously appearing adenocarcinoma and small-cell lung carcinoma harbouring an identical EGFR exon 19 mutation. Ann Oncol 2012;23:3188-9. [Crossref] [PubMed]
- Morinaga R, Okamoto I, Furuta K, et al. Sequential occurrence of non-small cell and small cell lung cancer with the same EGFR mutation. Lung Cancer 2007;58:411-3. [Crossref] [PubMed]
- Watanabe S, Sone T, Matsui T, et al. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer 2013;82:370-2. [Crossref] [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [Crossref] [PubMed]
- Moore AM, Einhorn LH, Estes D, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: a Hoosier Oncology Group phase II trial. Lung Cancer 2006;52:93-7. [Crossref] [PubMed]
- Lee JK, Lee J, Kim S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol 2017;35:3065-74. [Crossref] [PubMed]
- Meder L, König K, Fassunke J, et al. Implementing amplicon-based next generation sequencing in the diagnosis of small cell lung carcinoma metastases. Exp Mol Pathol 2015;99:682-6. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [Crossref] [PubMed]
- Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res 2014;20:1698-705. [Crossref] [PubMed]
- Roca E, Gurizzan C, Amoroso V, et al. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treat Rev 2017;59:117-22. [Crossref] [PubMed]
- Suda K, Murakami I, Sakai K, et al. Small cell lung cancer transformation and T790M mutation: complimentary roles in acquired resistance to kinase inhibitors in lung cancer. Sci Rep 2015;5:14447. [Crossref] [PubMed]
- List M, Jamous F, Gupta A, et al. Anti-hu positive antibodies and small cell carcinoma: a single center review. S D Med 2015;68:251, 253-5.
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017;377:2500-1. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018;33:853-61.e4. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]
- Iyer RR, Pluciennik A, Burdett V, et al. DNA mismatch repair: functions and mechanisms. Chem Rev 2006;106:302-23. [Crossref] [PubMed]
- Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]