
The lymphocyte-to-monocyte ratio could predict the efficacy of PD-1 inhibitors in patients with advanced cancer
Introduction
Tumor immunotherapy has been a popular area of research in the field of tumor therapy in recent years, especially the application of immune checkpoint inhibitors (ICIs), which has brought tumor immunotherapy into a new era. Unlike conventional treatment methods, such as chemotherapy, radiation, and targeted therapy, which directly affect tumor cells, ICIs stimulate tumor-specific T-cell functions by blocking immune checkpoint pathways in the tumor microenvironment, thereby improving endogenous antitumor immunity to achieve antitumor effect.
In recent years, ICIs, represented by PD-1/PD-L1 inhibitors, have made remarkable achievements in the field of tumor treatment. Because of to their wide antitumor applicability and long-lasting effects, it has become the most promising tumor immunotherapy strategy. PD-1/PD-L1 has become the standard treatment for multiple malignancies, including malignant melanoma, non-small cell lung cancer (NSCLC), Hodgkin lymphoma, squamous cell carcinoma of the head and neck, renal cell carcinoma, and so on. Although PD-1/PD-L1 monoclonal antibodies have achieved promising results in clinical antitumor applications, not all patients benefit from this method. The overall objective response rate (ORR) of patients receiving monotherapy with ICIs is 20% to 40% (1). Some patients (9–29%) experienced hyperprogressive disease after receiving anti-PD-1 antibody, that is, the patient’s tumor volume increased by 50% within 2 months after receiving anti-PD-1/PD-L1 antibody monotherapy, and the tumor growth rate was more than twice before treatment (2,3). In addition, the adverse reactions of PD-1/PD-L1 monoclonal antibodies cannot be ignored, and some of which are fatal, such as immune-related pneumonia and hepatitis (4). The unpredictable efficacy and high cost of treatment have prevented the widespread use of PD-1/PD-L1 inhibitor therapy. Therefore, finding effective and accurate biomarkers to predict the efficacy of ICIs and selecting potential clinical benefit populations are important areas of clinical research.
PD-L1 expression is the earliest clinical biomarker thought to predict PD-1/PD-L1 inhibitors (5). In many tumors, such as melanoma, NSCLC, renal cell carcinoma, and ovarian cancer, PD-L1 overexpression has been observed to have a significant correlation with the efficacy of PD-1/PD-L1 inhibitors (6-8). However, no correlation between nivolumab’s therapeutic effect and PD-L1 expression status has been found in lung squamous cell carcinoma (9). The same is true for renal cell carcinoma, which has median overall survival (OS) of 21.8 and 27.4 months for patients with PD-L1-positive expression ≥1% and <1%, respectively (10). This indicates that PD-L1 expression is still inaccurate as a biomarker for predicting efficacy, and the reasons include the following: (I) PD-L1 expression is heterogeneous and dynamic. There are differences in PD-L1 expression for different tumor sites, different stages of the disease, and different treatments. (II) Since PD-L1 can be expressed in tumor cells and immune cells at the same time, PD-L1 expressed by immune cells should also be included in the detection of PD-L1 by immunohistochemistry. (III) Different detection platforms, antibodies, and set positive thresholds affect the test results, leading to a significant reduction in the clinical significance of PD-L1 testing and may lead to inappropriate treatments (11). In addition, several other biomarkers have been reported, such as tumor mutation burden (12), CD8+ tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment (13), microsatellite instability-high or deficient mismatch repair (14), and specific inflammation and interferon-γ-related mRNA-based signatures (15), and so on, but research on these biomarkers remains in the experimental phase. Therefore, due to the inaccuracy of PD-L1 as a predictor and other indicators are still in the research stage, there is an urgent need to develop reliable, novel, and inexpensive markers to identify specific patients most likely to benefit from PD-1 inhibitors.
Previous studies have reported that peripheral blood cells, including neutrophils, lymphocytes, platelets, and monocytes, can promote tumor proliferation, invasion, and metastasis (16,17). Based on this evidence, many inflammatory indicators such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) have been used as indicators of prognosis for various tumors (18-22). At present, there have been some reports on the relationship between NLR in patients with PD-1 inhibitors and the prognosis of lung cancer, kidney cancer, and melanoma (23-25), but the LMR has been less studied in patients treated with PD-1 inhibitors. This retrospective study investigated the role of the LMR as a predictor of response to PD-1 inhibitor in patients with advanced cancer. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1451).
Methods
Patients and data collection
We retrospectively analyzed patients with refractory or recurrent advanced cancer who were treated with PD-1 inhibitors (Opdivo, Keytruda, or other drugs) at Henan Cancer Hospital from January 1, 2017, to July 31, 2019. Other inclusion criteria are as follows: received at least 2 PD-1 inhibitor treatment cycles; with at least one measurable lesion as defined by the solid tumor response assessment criteria (RECIST 1.1); completed clinical and follow-up information and clear prognosis; with blood routine data within 1 week (baseline) before PD-1 inhibitor treatment and 6±2 weeks after PD-1 inhibitor treatment; underwent baseline computer tomography or magnetic resonance imaging assessment (within 4 weeks before PD-1 inhibitor treatment); no uncontrolled infection.
The data collected included the age, sex, metastatic site of each patient; the treatment before PD-1 inhibitor application; the type of PD-1 inhibitor; ECOG performance status; smoking and drinking history; serum lactate dehydrogenase; the LMR in peripheral blood within 1 week before starting anti-PD-1 antibody treatment and 6±2 weeks after treatment initiation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital (No. ChiCTR1900024385), and informed consent was taken from all the patients.
Tumor evaluation
Tumor evaluation was performed every 6 weeks or whenever the patient had significant advanced symptoms. The evaluation was processed according to RECIST 1.1: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR was calculated as the percentage of CR or PR among all patients with the target lesion treated. OS time was defined as the time from initiation of PD-1 inhibitor until death (event) or last follow-up (censored). Progression-free survival (PFS) time was defined as time from initiation of PD-1 inhibitor until disease progression or death (event) or last follow-up (censored).
Statistical analysis
The LMR was defined as the ratio of lymphocytes to monocytes in peripheral blood, and LMR-week 6 was the LMR 6±2 weeks after the first application of anti-PD-1 antibody. The objective response (CR or PR) was used as the state variable, and the LMR was used as the test variable to construct the receiver operating characteristic (ROC) curve. The area under the curve (AUC) indicates the ability to predict tumor shrinkage. The maximum value of the Youden index was selected as the cutoff value of the LMR; then, the patients were divided into high- and low-LMR groups. Demographic characteristics are expressed as percentages for categorical variables and as medians and ranges for quantitative variables. The Kaplan-Meier method was used for survival analysis, and the log-rank test was used to compare the differences between the two groups. Cox proportional hazards model was used for univariate analysis, and factors with significant differences were included in multivariate analysis. SPSS version 21.0 (IBM Corp., Armonk, NY, USA) was used for the above statistical analysis. All statistical tests were two-sided, and significance was set at P≤0.05.
Results
Patient characteristics
This retrospective analysis included 121 patients receiving anti-PD-1 treatment, with an ORR of 19.0% (21/121 patients). The basic patients’ characteristics are shown in Table 1. Of the 121 patients, 40 had melanoma, 25 had renal cell carcinoma, 36 had liver cancer, and 20 had NSCLC.
Table 1
| Characteristic | Subgroup | N=121 |
|---|---|---|
| Age (years) | Median | 55.5 |
| Range | 20–82 | |
| LMR-1 | Median | 4.76 |
| IQR | 3.15–6.06 | |
| LMR-week 6 | Median | 3.91 |
| IQR | 2.63–5.75 | |
| Gender, n (%) | Female | 40 (33.1) |
| Male | 81 (66.9) | |
| No. of metastatic sites before PD-1 inhibitor therapy, n (%) | ≤2 | 59 (48.8) |
| >2 | 62 (51.2) | |
| Number of previous therapies, n (%) | 1 | 37 (30.6) |
| 2 | 51 (42.1) | |
| ≥3 | 33 (27.3) | |
| Type of inhibitor, n (%) | Keytruda | 31 (25.6) |
| Opdivo | 57 (47.1) | |
| Other | 33 (27.3) | |
| ECOG PS, n (%) | 0–1 | 92 (76.0) |
| ≥2 | 29 (24.0) | |
| Smoking, n (%) | Yes | 40 (33.0) |
| No | 81 (67.0) | |
| Drinking, n (%) | Yes | 37 (30.6) |
| No | 84 (69.4) |
IQR, interquartile range; LDH, lactic dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group performance status; No., number; PD-1, programmed death-1; LMR-1, lymphocyte-to-monocyte ratio baseline; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
Survival analysis
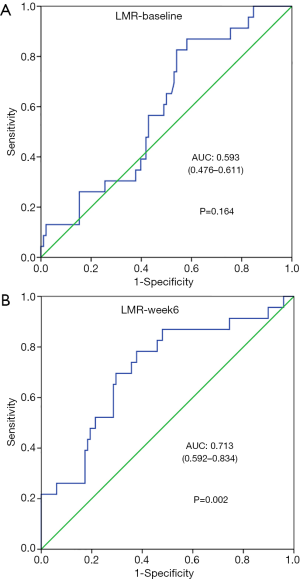
We collected LMR data within 1 week before the first PD-1 inhibitor treatment (LMR-week 1) and 6±2 weeks after the initiation of treatment (LMR-week 6). The ROC curve shows that the AUCs were 0.593 (P=0.164) (Figure 1A) and 0.713 (P=0.002) (Figure 1B) for LMR-week 1 and LMR-week 6, respectively, and LMR-week 1 was not statistically significant. The area under the curve of LMR-week 6 was slightly larger than that of LMR-week 1, which was related to the response of the tumor to the treatment (the optimal response CR or PR). There was no significant correlation between LMR-week 1 and the response of the tumor to the treatment (P>0.05). Based on the ROC curve, the LMR-week 6 cutoff value was 4.15, and the patients were divided into a high-LMR-week 6 group (LMR-week 6 >4.15) and a low-LMR-week 6 group (LMR-week 6 ≤4.15).
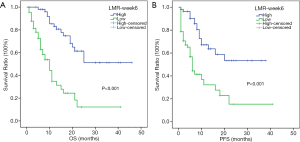
The Kaplan-Meier analysis showed that low LMR-week 6 was associated with poor OS and PFS in patients with advanced tumors (P<0.001) (Figure 2). The median OS of the low-LMR-week 6 group was 10.0 months [95% confidence interval (CI): 6.87–13.13], and the high-LMR-week 6 group had no median OS due to insufficient deaths (P<0.001) (Figure 2A); the median PFS in the low-LMR-week 6 group was 6 months (95% CI: 4.87–7.13), and there was no median PFS in the high-LMR-week 6 group (P<0.001) (Figure 2B).
After the univariate analysis, smoking history, ECOG performance status ≥2, and LMR-week 6 ≤4.15 were associated with poor OS and PFS (Table 2), but the multivariate analysis showed that the independent factors associated with OS reduction were ECOG performance status and LMR-week 6, whereas those associated with decreased PFS were smoking history, ECOG, and LMR-week 6. This indicated that patients with LMR-week 6 ≤4.15 had a 3.85-fold increased risk of death (hazard ratio: 3.851; 95% CI: 2.039–7.276; P<0.001), and a 2.67-fold increased risk of progression (hazard ratio: 2.67; 95% CI: 1.447–4.927; P=0.002) (Table 2).
Table 2
| Factors | Overall survival | Progression–free survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate HR, 95% CI | P value | Multivariate HR, 95% CI | P value | Univariate HR, 95% CI | P value | Multivariate HR, 95% CI | P value | ||
| Gender (male vs. female) | 1.788 (0.941–3.4) | 0.076 | 1.836 (0.966–3.489) | 0.064 | |||||
| Age (≥65 vs.<65) | 1.248 (0.713–2.184) | 0.438 | 1.278 (0.73–2.235) | 0.39 | |||||
| No. of metastatic sites (>2 vs.≤2) | 1.442 (0.837–2.483) | 0.187 | 1.52 (0.883–2.619) | 0.131 | |||||
| Smoking history (yes vs. no) | 1.98 (1.16–3.381) | 0.012 | 1.713 (0.986–2.975) | 0.056 | 2.055 (1.203–3.509) | 0.008 | 1.774 (1.027–3.062) | 0.04 | |
| Drinking history (yes vs. no) | 1.6 (0.929–2.755) | 0.09 | 1.721 (0.998–2.968) | 0.051 | |||||
| Lactate dehydrogenase (≥245 vs. <245) | 1.564 (0.894–2.735) | 0.117 | 1.56 (0.89–2.736) | 0.121 | |||||
| Number of previous therapies (1,2 vs. ≥3) | 1.82 (0.961–3.448) | 0.066 | 1.501 (0.8–2.815) | 0.206 | |||||
| Type of inhibitor (Keytruda, Opdivo vs. other) | 1.576 (0.848–2.929) | 0.165 | 1.538 (0.822–2.876) | 0.192 | |||||
| ECOG PS (≥2 vs. 0,1) | 3.865 (2.237–6.677) | <0.001 | 2.36 (1.326–4.202) | 0.004 | 4.187 (2.403–7.294) | <0.001 | 2.862 (1.597–5.128) | <0.001 | |
| LMR-6 week (≤4.15 vs. >4.15) | 4.705 (2.572–8.607) | <0.001 | 3.851 (2.039–7.276) | <0.001 | 3.373 (1.873–6.073) | <0.001 | 2.67 (1.447–4.927) | 0.002 | |
HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; No., number; PD-1, programmed death-1; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
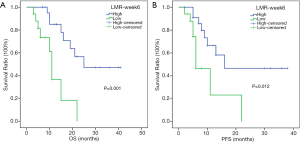
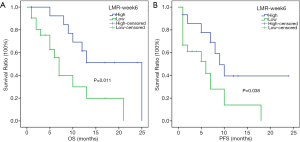
In the subgroup analysis, the respective median OSs of melanoma patients in the high- and low-LMR-week 6 groups were 25 (95% CI: not reached) and 11 months (95% CI: 9.713–12.287 months) (P=0.001) (Figure 3A), whereas the respective median PFSs were 16 (95% CI: not reached) and 6 months (95% CI: 2.663–9.337 months) (P=0.012) (Figure 3B). For hepatic carcinoma, the respective median OSs in the high- and low-LMR-week 6 groups were 25 (95% CI: not reached) and 7 months (95% CI: 5.421–8.579 months) (P=0.011) (Figure 4A), whereas the median PFSs in the high- and low-LMR-week 6 groups were 9 (95% CI: 6.02–11.98 months) and 6 months (95% CI: 0.452–11.548 months) (P=0.038) respectively (Figure 4B).
Response
In the low-LMR-week 6 group, 1 (1.5%) patient had CR, 4 (6.1%) patients had PR, 22 (33.3%) patients had SD, and 39 (59.1%) patients had PD. In the high-LMR-week 6 group, 7 (12.7%) patients had CR, 11 (20%) patients had PR, 12 (21.8%) patients had SD, and 25 (45.5%) patients had PD (Table 3). The chi-square test showed that the ORRs of the high- and low-LMR-week 6 groups were 32.7% (18/55) and 7.6% (5/66) (P<0.001), respectively. A higher LMR-week 6 was independently associated with a higher ORR (P<0.001) (Figure 5). The low- and high-LMR-week 6 ORRs were 0% and 13% (Table 4), respectively, in 40 melanoma patients; 9.5% and 33.4%, respectively, in 36 hepatic carcinoma patients (Table 5); 13.4% and 60%, respectively, in 25 renal cell carcinoma patients (Table 6); and 7.7% and 57.25%, respectively, in 20 NSCLC patients (Table 7).
Table 3
| Response | Number (N=121, %) | Low LMR-week 6 (N=66, 54.5%) | High LMR-week 6 (N=55, 45.5%) |
|---|---|---|---|
| CR | 8 (6.6) | 1 (1.5) | 7 (12.7) |
| PR | 15 (12.4) | 4 (6.1) | 11 (20.0) |
| SD | 34 (28.1) | 22 (33.3) | 12 (21.8) |
| PD | 64 (52.9) | 39 (59.1) | 25 (45.5) |
N, number; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
Table 4
| Response | Number (N=40, %) | Low LMR-week 6 (N=17, 42.5%) | High LMR-week 6 (N=23, 57.5%) |
|---|---|---|---|
| CR | 2 (5.0) | 0 (0.0) | 2 (8.7) |
| PR | 1 (2.5) | 0 (0.0) | 1 (4.3) |
| SD | 16 (40.0) | 9 (52.9) | 7 (30.5) |
| PD | 21 (52.5) | 8 (47.1) | 13 (56.5) |
N, number; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
Table 5
| Response | Number (N=36, %) | Low LMR-week 6 (N=21, 58.3%) | High LMR-week 6 (N=15, 41.7%) |
|---|---|---|---|
| CR | 1 (2.8) | 0 (0.0) | 1 (6.7) |
| PR | 6 (16.7) | 2 (9.5) | 4 (26.7) |
| SD | 9 (25.0) | 6 (28.6) | 3 (20.0) |
| PD | 20 (55.5) | 13 (61.9) | 7 (46.6) |
N, number; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
Table 6
| Response | Number (N=25, %) | Low LMR-week 6 (N=15, 60%) | High LMR-week 6 (N=10, 40%) |
|---|---|---|---|
| CR | 4 (16.0) | 1 (6.7) | 3 (30.0) |
| PR | 4 (16.0) | 1 (6.7) | 3 (30.0) |
| SD | 8 (32.0) | 7 (46.6) | 1 (10.0) |
| PD | 9 (36.0) | 6 (40.0) | 3 (30.0) |
N, number; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
Table 7
| Response | Number (N=20, %) | Low LMR-week 6 (N=13, 65%) | High LMR-week 6 (N=7, 35%) |
|---|---|---|---|
| CR | 1 (5.0) | 0 (0.0) | 1 (14.3) |
| PR | 4 (20.0) | 1 (7.7) | 3 (42.9) |
| SD | 1 (5.0) | 0 (0.0) | 1 (14.3) |
| PD | 14 (70.0) | 12 (92.3) | 2 (28.6) |
NSCLC, non-small-cell lung cancer; N, number; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; LMR-week 6, lymphocyte-to-monocyte ratio 6 weeks after the start of PD-1 inhibitors.
Discussion
This study investigated the prognostic value of the LMR in patients with advanced tumors using PD-1 inhibitors, and results showed that in patients receiving PD-1 inhibitors, there was no significant correlation between LMR-week 1 and tumor response to treatment (AUC: 0.593, P>0.05), but LMR at week 6 after treatment was significantly correlated with tumor response to treatment (AUC: 0.713 P=0.002). In addition, an increase in LMR-week 6 was associated with better PFS, OS, and ORR, suggesting that LMR-week 6 may be a biomarker to stratify patients who might have a better response to PD-1 inhibitors.
The inflammatory response plays a key role in different stages of tumor progression, including initiation, proliferation, invasion, metastasis, and angiogenesis (26,27). Lymphocytes, as a major component of the immune system, can inhibit tumor proliferation and metastasis through cytotoxicity, thereby participating in tumor surveillance and defense (28). Monocytes are also the body’s main immune cells. They change the tumor microenvironment through local immunosuppression and their effect on angiogenesis, thereby promoting the progression of cancer (29). Studies have confirmed that a high monocyte count is one of the factors with poor prognosis in patients with malignant tumors (30,31).
In addition, a series of studies have shown that a low LMR is associated with poor prognosis in many cancers. However, the mechanism by which LMR affects the prognosis of tumor patients has not been clarified. TILs and tumor-associated macrophages (TAMs) have been found in a variety of malignant tumor tissues and can play a precise role in predicting prognosis (32). TILs control the progression of tumors by participating in the body’s cellular and humoral immunity. Low lymphocytes counts reduce the body’s inhibitory effect on tumor cells, leading to poor prognosis. TAMs are monocytes originating in circulating blood and are active around tumor tissues for tumor chemokine secretion. At the same time, TAMs can promote tumor angiogenesis and produce anti-immune responses by producing growth factors and cytokines to accelerate tumor progression. Studies have confirmed that patients with high TAM infiltration have poor prognosis (33). The absolute value of peripheral blood mononuclear cells is a biological marker that can replace TAMs. Therefore, LMR can be used as a biomarker of comprehensive lymphocytes and monocytes to evaluate the prognosis of patients with malignant tumors. As an indicator of inflammatory response, LMR has the characteristics of simple, fast, operable, specific and sensitive detection methods and may be considered as an early alternative indicator for predicting the outcome of patients receiving anti-PD-1 agents. At present, some studies have reported the relationship between the NLR and prognosis of lung cancer, kidney cancer, and melanoma in patients with PD-1 inhibitors (23-25). The relationship between the LMR and the prognosis of patients treated with anti-PD-1 agents is less well studied.
There are some limitations to our study. First, the study included various types of cancer, and the number of patients with each type of cancer was limited. Second, this was a retrospective study, so there may have been a risk of bias and confounding factors. In addition, the follow-up time was short, and there were not enough death data in the high-LMR group; thus, the median survival time could not be calculated. Therefore, prospective, randomized, multicenter clinical trials with larger sample sizes are needed in future studies to provide more reliable data.
Conclusions
The higher LMR at the sixth week after the initiation of PD-1 inhibitors is significantly related to the therapeutic effect. Therefore, LMR-week 6 can be used as an early substitute indicator for stratification in patients with a better response to anti-PD-1 agents, which will help clinicians determine individualized treatment planning as well as avoid adverse reactions and increase response rates.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1451
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1451
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1451). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital (No. ChiCTR1900024385), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iwai Y, Hamanishi J, Chamoto K, et al. Cancer immunotherapies targeting the PD- 1 signaling pathway. J Biomed Sci 2017;24:26. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847-56. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Kefford R, Ribas A, Hamid O, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. British Journal of Special Education 2014;19:136.
- Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C), an anti- PD- L1 antibody, in patients with recurrent or refractory ovarian cancer: a phase Ib trial reporting safety and clinical activity. European Cancer Congress 2015;51:31515.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Sheng XL, Cheng SF, Yu JJ, et al. Therapeutical biomarkers for PD-1/PD-L1 inhibitors. Chin J Cancer Biother 2019;26:104-11.
- Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377:2500-1. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site-when a biomarker defines the indication. N Engl J Med 2017;377:1409-12. [Crossref] [PubMed]
- Schalper KA, Kaftan E, Herbst RS. Predictive biomarkers for PD-1 Axis therapies: the hidden treasure or a call for research. Clin Cancer Res 2016;22:2102-4. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Coffelt SB, de Visser K. Cancer: Inflammation lights the way to metastasis. Nature 2014;507:48-9. [Crossref] [PubMed]
- Zhang X, Zhang W, Feng LJ. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One 2014;9:e111906. [Crossref] [PubMed]
- Yang JJ, Hu ZG, Shi WX, et al. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a metaanalysis. World J Gastroenterol 2015;21:2807-15. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58. [Crossref] [PubMed]
- Abe S, Kawai K, Nozawa H, et al. LMR predicts outcome in patients after preoperative chemoradiotherapy for stage II-III rectal cancer. J Surg Res 2018;222:122-31. [Crossref] [PubMed]
- Lalani AA, Xie W, Martini DJ, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer 2018;6:5. [Crossref] [PubMed]
- Zer A, Sung MR, Walia P, et al. Correlation of Neutrophil to Lymphocyte Ratio and Absolute Neutrophil Count With Outcomes With PD-1 Axis Inhibitors in Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:426-34.e1. [Crossref] [PubMed]
- Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet 2001;357:539-45. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Chanmee T, Ontong P, Konno K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670-90. [Crossref] [PubMed]
- Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophi land monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 2005;93:273-8. [Crossref] [PubMed]
- Lee YY, Choi CH, Sung CO, et al. Prognostic value of pretreatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecol Oncol 2012;124:92-7. [Crossref] [PubMed]
- Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer 2013;4:84-95. [Crossref] [PubMed]
- Tsutsui S, Yasuda K, Suzuki K, et al. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 2005;14:425-31. [PubMed]


