
Intraoperative radiotherapy during kyphoplasty (Kypo-IORT): a novel treatment approach for patients with symptomatic spinal metastases
Introduction
Approximately 30-40% of all cancers patients develop bone metastases (1). In Germany alone, there are about 50,000 patients with bone metastases per year. Up to 50% of the osseous metastases are located in the vertebral column, most frequently located in the thoracic spine (70%), followed by the lumbar spine (20%) (2). In our own patient cohort, we have seen similar results. We analysed 53 patients, who received an external beam radiation for vertebral metastases during September 2007 and March 2008. Overall there were 356 vertebral metastases, 200 (56%) located in the thoracic spine, 105 (30%) located in the lumbar spine and 51 (14%) located in the cervical spine (3). Common complications of vertebral metastases are axial pain, pathological fracture and neurological dysfunction by spinal cord compression. Although the median overall survival of patients with bone metastases is only 7-9 months, an increase of life expectancy has been seen recently due to better oncologic treatment (4-6). Therefore enhanced therapy regimes for patients with bone metastases are necessary to realize a high quality of life. External beam radiotherapy (EBRT) is thought to be the gold standard in patients with symptomatic vertebral metastases and dose fraction schedules vary from 8 Gy in one fraction to 20 Gy in five fractions, 30 Gy in ten fractions and 40 Gy in 20 fractions (7). EBRT is effective for pain relief after a few days with good local control rates (8,9). Koswig et al. described pain response rates between 78-81% after single fraction radiotherapy (1×8 Gy) and fractionated EBRT (10×3 Gy) without significant difference in both treatment arms (10). However recalcification of bone metastases takes up to six months with persisting risk for fracture during this time. Therefore surgical interventions like vertebral augmentation are often useful to reach an immediate stabilization. As such techniques have no anticancer effect postoperative radiotherapy is given to prevent early regrowth. This leads to treatment periods of two to four weeks.
A possibility to shorten treatment time is spine stereotactic body radiotherapy (SBRT) with one to five dose fractions. SBRT also provides excellent local control and pain response comparable or even better than standard EBRT (11,12). Another opportunity to shorten treatment time is one-stage-procedures like kyphoplasty combined with other physical methods (13,14). We developed a novel approach to deliver intraoperative radiotherapy during kyphoplasty (Kypho-IORT) (3). Schneider et al. showed that about 30% of the patients, who received an EBRT for spinal metastases are suitable for Kypho-IORT (3). In general patients with pathologically or cytologically proven primary tumor and painful or instable lesions between thoracic level 4 and lumbar level 5 are eligible for Kypho-IORT.
Patients with primary bone tumor, soft tissue invasion or epidural space/intraspinal invasion are not suitable for this new treatment approach.
The procedure of Kypho-IORT was described in detail before (15-17). In short, under general anesthesia the patients were placed in prone position and a bipedicular approach was chosen. A specially designed needle applicator (outer diameter: 4.2 mm) and specially designed and multiply modified metallic sleeves were developed to enable the use of the Intrabeam® system for Kypho-IORT. The sleeve and the applicator protect the drift tube of the Intrabeam® system against bending stress. The sleeves were inserted transpedicularly into the vertebrae and afterwards the needle applicator including the drift tube was guided through the sleeves into the metastases. After verification of the applicator position by biplanar X-ray the IORT was delivered. After irradiation the drift tube was removed and standard kyphoplasty with minor modifications was done. To exclude any surgical complications all patients received a X-ray of the spine at the first postoperative day.
Case report 1
A 58-year-old woman with primary osseous and hepatic metastasizing colon cancer was presented with painful metastasis of the 7th thoracic vertebra (Figure 1). The diagnosis was histologically confirmed one month ago. The magnetic resonance imaging (MRI) showed a pathological fracture of the 7th thoracic vertebra classified as unstable. No further bone metastases were detected.
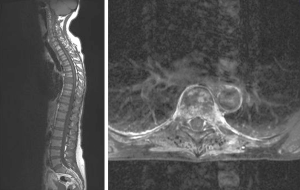
Because of the instability and the intensity of pain measured by the visual analogue scale (VAS 7/10) we decided to perform a Kypho-IORT.
Kypho-IORT was done as described above. A radiation dose of 8 Gy in 8 mm distance from the isocenter of the radiation source was delivered during about 80 seconds to the center of the metastasis. The whole procedure lasted 50 minutes. One day after surgery there was an obvious pain reduction from preoperatively VAS 7/10 to VAS 2/10. No intra- or postoperative complication occurred. No radiation induced skin reaction was seen. This one-step approach realized an immediate beginning of the required chemotherapy and only a few days after the patient received the first of five cycles chemotherapy with FOLFIRI/Avastin.
At the first follow-up 3 months after Kypho-IORT the patient was pain free. The scar was nonirritated (Figure 2). There were no radiation induced skin toxicities. MRI showed a stable situation of the 7th thoracic vertebra (Figure 3), but new metastases of the 12th thoracic and 1st and 2nd lumbar vertebra with pathological depressed fracture of the base and upper plates. Therefore the patient received EBRT from 11th thoracic vertebra to the 3rd lumbar vertebra with 30 Gy. Unfortunately the patient died 3 months later due to rapid systemic disease progression.
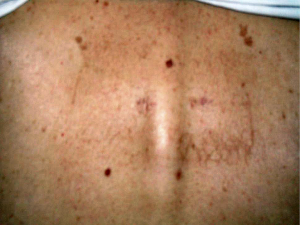
Case report 2
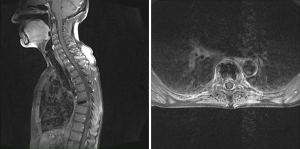
A 70-year-old woman presented with receptor-positive ductal-invasive breast cancer and simultaneous solitary spinal metastasis of the 6th thoracic vertebra [stage pT2 pN1 (2/11) M1 (bone); her2neu negative] (Figure 4). There was severe axial pain (VAS 6/10). This case was discussed in a multidisciplinary team meeting and management of the malignancy included breast conserving surgery, radiotherapy of the spinal metastasis and adjuvant chemotherapy. Therefore she previously underwent quadrantectomy and axillary dissection. A few days later Kypho-IORT of the 6th thoracic vertebra was done, delivering a radiation dose of 8 Gy in 8 mm distance from the isocenter of the radiation source. The whole surgery lasted 63 minutes. No intra- or perioperative complications were seen. There was no radiation induced skin toxicity. First day after Kypho-IORT there was a significant local pain improvement (VAS 0/10). The planned adjuvant chemotherapy with three cycles of FEC and three cycles of docetaxel was started. Moreover an antiresoptive therapy was initiated. At the first follow-up 6 weeks after Kypho-IORT the patient was free of pain (VAS 0/10) without any pain medication. The scar was non-irritated. There was no radiation related skin toxicity, neither radiologically assessed local progression.
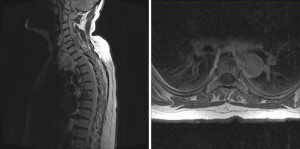
After chemotherapy the patient received hypofractionated whole breast radiotherapy (40.05/2.67 Gy, KOSIMA trial NCT 01403779) followed by endocrine therapy using tamoxifen. The further quarterly follow-ups showed no pain recurrence or radiologically assessed progression of the malignancy. After 21 months the patient again suffered from severe axial pain (VAS 6/10) and the computed tomography (CT) imaging showed newly diagnosed metastases of the 2nd, 3rd and 4th lumbar vertebra with stable disease of the 6th thoracic vertebra. The 4th vertebra showed an increased risk for a pathological fracture (Figure 5). Therefore we again decided to do a Kypho-IORT of the 4th lumbar vertebra. A radiation dose of 8 Gy in 13 mm distance from the isocenter of the radiation source was delivered. The whole procedure lasted 36 minutes. Again no complications or radiation induced skin toxicities were seen. The patient had a significant pain reduction (VAS 1/10) one day after Kypho-IORT Afterwards EBRT (3 Gy per day, total dose 30 Gy) from the 1st to the 3rd lumbar vertebra was given and endocrine therapy was switched from tamoxifen to letrozole.
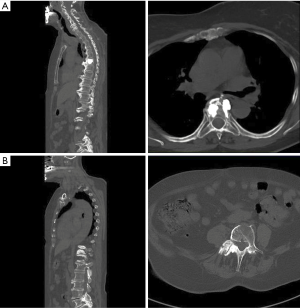
At the last follow-up 3 months after Kypho-IORT the CT of the total spine and the bone scan showed a stable disease without indication of local recurrence. No radiation induced skin toxicity was seen. The patient was nearly pain-free (thoracic spine: VAS 0/10; lumbar spine: VAS 1/10).
Discussion
Although due to better oncologic treatment longer life expectancy of patients with bone metastases is seen, treatment aims mainly at palliation (pain relief, mechanical stability) and local disease control. To shorten treatment time and reduce hospitalization time, alternative treatment approaches to primary or postoperative normofractionated EBRT are more often used. One possibility is SBRT. For SBRT a comprehensive review suggested local control rates of 77-100% from around 900 metastases, in patients who are radiation native, previously radiated or postoperative with or without previous radiation exposure (11).
One of the major complications after SBRT is SBRT-induced vertebral compression fracture which occurs in approximately 10% (12,18,19). Cunha et al. (18) reports vertebral compression fracture rates of 11%, 12 de novo fractures (63%) and seven cases of fracture based on an existing fracture at the site of treatment after SBRT (37%). Median time to fracture after SBRT was 3.3 months. Doses per fraction of 20 Gy or greater were significant predictors of vertebral compression fractures. About half of the patients needed a salvage surgical intervention typically done as cement augmentation procedures like balloon kyphoplasty or vertebroplasty.
Other possibilities to reduce overall treatment time are, e.g., kyphoplasty combined with intravertebral administration of 153Samarium or interstitial implantation of 125I seeds (13,14). In comparison to the above mentioned treatment strategies Kypho-IORT has some important advantages.
Although we also apply a high single dose (91 Gy at the applicator surface, 45 Gy in 1 mm depth from the applicator surface) in contrast to the SBRT technique we can avoid radiation induced vertebral compression fracture due to the simultaneously done kyphoplasty. Moreover this approach allows histological sampling with molecular subtyping, so further systemic therapies (e.g., antihormonal therapy, targeted therapy) can be adjusted accordingly. In comparison to the above mentioned techniques with radioactive material, this X-ray based approach can be done in a standard operating room and the risks of open radionuclides and leakage of radioactive material can be avoided.
Our recently published preliminary data of this novel treatment approach showed technical feasibility and significant pain relief with good local tumor control (15-17,20). Based on these results we have done a dose escalation study (NCT 01280032) to establish the maximum tolerable dose (MTD) of Kypho-IORT. The institutional review board of the University of Heidelberg and the Federal Office for Radiation Protection (Bundesamt für Strahlenschutz) reviewed and approved the protocol and the study was performed in accordance with the Declaration of Helsinki. For dose escalation there were three planned dose levels: 8 Gy in 5 mm, 8 Gy in 8 mm and 8 Gy in 10 mm depth from the applicator surface (≙8 Gy in 8 mm, 8 Gy in 10 mm and 8 Gy in 13 mm depth from the isocenter of the radiation source). Between the different dose levels we had a 90 day surveillance interval to exclude potential dose-limiting toxicities (DLTs). DLTs were defined as wound healing problems, infections, osteoradionecrosis, nerve/spinal cord damage and pathological fractures within the above mentioned 90 day interval. We planned to treat three patients at the given dose level of 8 Gy in 8 mm and follow up for at least 90 days and then escalate to the next dose level if no DLTs occurred. If one patient developed a DLT, then up to three additional patients were to be treated at the same dose. If no DLT occurred in these three patients, dose was increased to the next higher level. However, if one out of these additional patients showed DLT, IORT for the next six patients was given at the next lower dose level.
Study inclusion criteria were: age ≥50 years, Karnofsky-index ≥60%, histologically or by imaging proven spinal metastases (2 cm diameter as the upper limit) caudal the third thoracic vertebrae accessible for Kypho-IORT.
Patients were not eligible if they had primary bone tumors, soft tissue invasion or epidural space invasion and tumor infiltration in the dorsal structures of the vertebrae (pedicle, lamina, etc.). Further exclusion criteria were: previous local treatment (irradiation, surgery), contraindication against anaesthesia or surgery, contraindication against MRI and CT examinations, uncontrolled concurrent illness (e.g., local infections of spine or the skin) or patients unable to consent.
Until now overall 80 patients were treated with Kypho-IORT for spinal metastases. Nine patients were included in the phase II dose escalation study. No DLTs were seen in one of the three dose levels. Therefore a dose of 8 Gy in 13 mm depths from the isocenter could be determined as a save maximally tolerated dose and will be consolidated in a phase III study.
Acknowledgments
Funding: Carl Zeiss Meditec AG supports radiobiological research at the University Medical Center Mannheim.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Intraoperative Radiotherapy II”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.03.01). The series “Intraoperative Radiotherapy II” was commissioned by the editorial office without any funding or sponsorship. FW served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resol
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coleman RE. Metastatic bone disease. Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76. [PubMed]
- Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin 2008;58:245-59. [PubMed]
- Schneider F, Greineck F, Clausen S, et al. Development of a novel method for intraoperative radiotherapy during kyphoplasty for spinal metastases (Kypho-IORT). Int J Radiat Oncol Biol Phys 2011;81:1114-9. [PubMed]
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-09. [PubMed]
- Yarnold YR. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Bone pain trail working party. Radiother Oncol 1999;52:111-21. [PubMed]
- Ratanatharathorn V, Powers WE, Moss WT, et al. Bone metastases: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys 1999;44:1-18. [PubMed]
- Souchon R, Wenz F, Sedlmayer F, et al. DEGRO practice guidelines for palliative radiotherapy of metastatic breast cancer: bone metastases and metastatic spinal cord compression (MSCC). Strahlenther Onkol 2009;185:417-24. [PubMed]
- Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine 2009;34:S78-92. [PubMed]
- van der Linden YM, Steenland E, van Houwelingen HC, et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: results on survival in the Dutch Bone Metastasis Study. Radiother Oncol 2006;78:245-53. [PubMed]
- Koswig S, Budach V. Remineralization and pain relief in bone metastases after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study. Strahlenther Onkol 1999;175:500-8. [PubMed]
- Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 2011;14:151-66. [PubMed]
- Sheehan JP, Shaffrey CI, Schlesinger D, et al. Radiosurgery in the treatment of spinal metastases: tumor control, survival, and quality of life after helical tomotherapy. Neurosurgery 2009;65:1052-61; discussion 1061-2. [PubMed]
- Ashamalla H, Cardoso ER, Macedon M, et al. Phase I trial of vertebral intracavitary cement and samarium (VICS): novel technique for treatment of painful vertebral metastasis. Int J Radiat Oncol Biol Phys 2009;75:836-42. [PubMed]
- Yang Z, Yang D, Xie L, et al. Treatment of metastatic spinal tumors by percutaneous vertebroplasty versus percutaneous vertebroplasty combined with interstitial implantation of 125I Seeds. Acta Radiol 2009;50:1142-48. [PubMed]
- Reis T, Schneider F, Welzel G, et al. Intraoperative radiotherapy during kyphoplasty for vertebral metastases (Kypho-IORT): first clinical results. Tumori 2012;98:434-40. [PubMed]
- Schmidt R, Wenz F, Reis T, et al. Kyphoplasty and intra-operative radiotherapy, combination of kyphoplasty and intra-operative radiation for spinal metastases: technical feasibility of a novel approach. Int Orthop 2012;36:1255-60. [PubMed]
- Wenz F, Schneider F, Neumaier C, et al. Kypho-IORT – A novel approach of intraoperative radiotherapy during kyphoplasty for vertebral metastases. Radiat Oncol 2010;5:11. [PubMed]
- Cunha MV, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys 2012;84:e343-9. [PubMed]
- Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 2009;27:5075-79. [PubMed]
- Bludau F, Schmidt R, Schneider F, et al. Learning and teaching abilities of a newly inaugurated operation technique. Analysis of learning curves and transferability exemplified by Kypho-IORT. Orthopade 2013;42:772-9. [PubMed]


