
An experimental study on the prosthesis produced by inactivated autologous subcutaneous adipose tissue and skin dermal tissue in a mini-pig model
Introduction
Breast cancer is one of the most common malignant tumors in women, with annually increasing incidence (1,2). Partial or total loss of one breast or even bilateral breast caused by operation for breast cancer patients not only results in physiological defects, but also brings great psychological burden. Moreover, it seriously decreases the quality of life and mental health of patients (3). At present, breast reconstruction techniques mainly included breast reconstruction based on autologous tissue transplantation, prosthesis implantation-based breast reconstruction, and breast reconstruction using autologous tissue with prosthesis (4). The pedicle rectus abdominis muscle flap and latissimus dorsi muscle flap were commonly used for breast reconstruction based on autologous tissue (5-7). In recent years, autologous adipose tissue transplantation has attracted more and more attention in the field of aesthetic plastic surgery (8,9). However, due to insufficient blood supply and support from other tissue structures, adipose tissue injected into the breast are absorbed and liquify easily, and the cosmetic and plastic effects are not lasting. As a result, repeated transplants are usually necessary. Meanwhile, siphoned adipose tissue could not be made into the conformal prosthesis for breast reconstruction in patients with partial breast loss (8,10,11). The authors hypothesized that heat-inactivated autologous adipose tissue wrapped in a dermal outer capsule can make a breast-like prosthesis for partial or total breast reconstruction. In order to test this hypothesis, in vitro and in vivo experiments were carried out to observe the morphological and histopathologic changes after implantation. We present the following article/case in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tcr-19-2844).
Methods
Animal and devices
The study was approved by the Ethics Committee of Zhejiang Cancer Hospital (No 2018-186). All animal tests were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (12) and efforts were conducted to minimize the number of animals used and any discomfort experienced. The animal tests were conducted in the animal experimental research center, Zhejiang University of Traditional Chinese Medicine from April-June 2018.
Six adult Bama female mini-pigs weighing 15.5 to 20 kg were purchased from Shanghai Jiagan Biotechnology Company Limited (Co., Ltd) [SCXK (Shanghai) 2015-0005, Shanghai, China] and individually reared in the Animal Experiment Research Center at Zhejiang University of Traditional Chinese Medicine [SYXK (Zhejiang) 2013-0184].
Laboratory feeding conditions include an ambient temperature of 20–22 °C, relative humidity of 40–70%, alternating 12 hours of light and dark environment, and full-price pellet feed twice a day. The equipment used in the laboratory included a Summit-gas anaesthetized respirator (Summit company, USA), Leica pathology slice (RM2245, Leica Inc., USA), and an automatic pathological scanning instrument (Nanozoomer S210, Hamamatsu Corporation, Japan).
In vitro experiment
The mini-pigs were adaptively fed for two weeks, and were then weighed after a 12-hour water fast. Before the operation, 4 mg/kg of ibuprofen tablets were administrated orally for preoperative analgesia. Trachea cannula was inserted after the administration of compound anesthesia with a Fumigation new II injection (0.2 mg/kg) and propofol (2 mg/kg), and the Summit-gas anaesthetized ventilator was connected with the trachea cannula. The anesthesia was maintained using 1–2% isoflurane. The skin of the back and the ventral side was shaved, and the routine area was sterilized and prepared. The back adipose tissue and the ventral dermis were removed under aseptic conditions. The in vitro experiments in the following six groups were carried out and repeated: (I) the adipose tissue and the dermis were immediately put into a formalin bath and fixed after removal at normal temperature (control fat, CF group); (II) the adipose tissue was put into a formalin bath and immobilized after treatment with water bath at 37 °C for 30 min (Pure fat, PF group); (III) the adipose tissue was fixed in formalin after a water bath at 56 °C for 30 min (inactivated fat, IF group); (IV) the adipose tissue and the dermis were immediately placed in a formalin bath and fixed after removal at normal temperatures (control fat plus dermis, CFD group); (V) the adipose tissue and dermis were fixed in formalin after a water bath at 37 °C for 30 min (Pure fat plus dermis, PFD group); (VI) the adipose tissue and the dermis were fixed in formalin after a water bath at 56 °C for 30 min (inactivated fat plus dermis, IFD group). After the tissue samples were fixed for 24 h, conventional dehydration, embedding, slicing and HE staining were done. The histological changes of the autologous adipose tissue or autologous adipose tissue with the outer capsule of the dermis were observed. Next, 100 adipocytes in each specimen were randomly selected. The diameter of the adipocytes were measured with Image Pro Plus 6th software (Media Cybernetics, USA), and the distributions of different adipocytes were analyzed.
In vivo experiment
Prosthesis preparation
The skin of the back and ventral side was shaved, and the routine area was sterilized and prepared. The specified volume of subcutaneous adipose tissue and the specified size of skin on the back were removed under aseptic conditions. The epidermis of the skin was extracted, and the dermis was retained and sutured into a prosthesis capsule. The autologous subcutaneous adipose tissue was placed into the capsule, and the capsule was sutured into a specified volume of prosthesis. The prosthesis was sterilized.
The prostheses were divided into four groups according to the different ways of administering autologous adipose tissue and dermis: pure autologous adipose tissue (PF group), inactivated autologous adipose tissue (IF group), pure autologous adipose tissue with the dermal outer capsule (PFD group) and inactivated autologous adipose tissue with the dermal outer capsule (IFD group).
Implantation method
In each mini-pig, 12 sides of the abdominal wall near the nipple were chosen. The prostheses were implanted into the subcutaneous tissue of the abdominal wall to form observable breast like shapes. Then, the incisions were sutured. After the operation, the mini-pigs were administrated oral ibuprofen tablets (4 mg/kg) and the skin incisions were smeared with ketoprofen gel for 3 days. During this period, 800 thousands units of penicillin sodium were injected for 3 days.
Observation and extraction
We observed the general situation of the mini-pigs after operation, including their mental state, diet, local state of implanted sites, and the appearance and texture of implants at the implanted sites. The presence of any acute and chronic inflammatory reactions such as exudation, fibrous tissue hyperplasia, encapsulation, contracture, sclerosis or calcification, etc. were observed. At the same time, the prostheses and their surrounding local tissues were removed at the 14th and 30th days after operation.
Histopathologic changes
Fixed tissues were dehydrated and embedded in paraffin blocks from which 4-um-thick sections were serially cut. Sections were stained with hematoxylin and eosin (H&E) as standard. The histopathological changes of prostheses and their surrounding tissues were observed including inflammatory cell infiltration and histologic changes of prostheses.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Analysis of Variance (ANOVA) single factor analysis of variance and multiple comparison of Tukey were used in this study. Differences were considered significant when P<0.05. Statistical analyses were conducted using Graphpad Prism 6 software (Graphpad software, USA).
Results
Outcomes of in vitro experiment
HE histopathological observation was performed after different treatments of autologous adipose tissue with or without external dermal capsule. The results showed that there were no significant changes in the size and histomorphology of autologous adipose tissue after different treatments, but the adipose tissue structure inactivated by heating was the clearest among them (Figure 1). In addition, upon examination of the dermal histopathology, there was no obvious abnormality in dermal structure in the PFD group compared with the FD group, but the dermal interstitium in the FID group was compact or slightly broken. Furthermore, the basic structure was still acceptable (Figure 1).
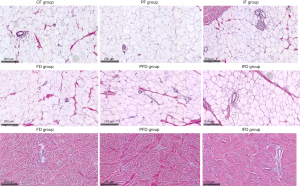
Quantitative analyses of the size of the adipocytes were performed for autologous subcutaneous adipose tissue using different treatments in vitro. There were no significantly statistical differences in distribution and average size of autologous adipose tissue among six groups (P>0.05, Figure 2).
Results of in vivo experiments
The skin appearance at implanted sites of mini-pigs and the general observation of the prostheses
On the first 3 days after operation, the diet and drinking water of the mini-pigs were not abnormal, the spirit was general, and the activities were mostly standing or suppressing. The skin in the surgical area was slightly red, but no obvious abscesses or dehiscence was found. At the 7th day after operation, the mental state of the mini-pigs had obviously improved. There were more “arch” activities, and the wounds were partially healed and dried.
At the 14th day after operation, the wound healed well in the surgical areas. Compared with before the operation, the skins of the mini-pigs in the PF and IF groups were slightly elevated, while those in the PFD and IFD groups were more obviously elevated. After the removal of the prostheses, a large number of fibrous connective tissues were found to be hyperplastic and wrapped around the prosthesis in the PF group. There was a moderate degree of fibrous connective tissue hyperplasia around the prosthesis in the IF group. On the other hand, there was less proliferation of fibrous connective tissues around the prostheses in the PFD and IFD groups (Figure 3).
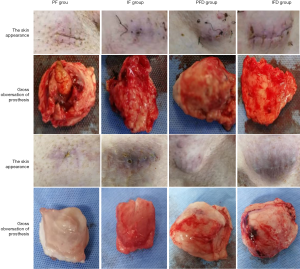
At the 30th day after operation, the wounds healed well at the implant sites. Compared with pre-implantation, the skin protuberances in the PFD and IFD groups were maintained or more prominent, and the IFD group had the best cosmetic effect. The prosthesis in the IFD group was slightly elastic, and its elasticity was not as soft as that of normal adipose tissue. After the prostheses were harvested, it was found that the prostheses in the PF and FI groups were obviously contracted or absorbed and were surrounded by a large number of fibrous connective tissues. On the other hand, there was less fibrous connective tissue proliferation around the prostheses in the PFD and FID groups (Figure 3).
Histopathological observation of implant site
At the 14th day after implantation, adipocytes in the prostheses of the PF and IF groups were gradually absorbed and replaced by proliferative fibrous connective tissues, while a large number of infiltrated inflammatory cells were found. In the PFD group, a small number of proliferative fibrous connective tissues and infiltrated inflammatory cells were observed in the adipocytes wrapped by dermis. Moreover, the morphology and structure of the adipocytes were normal. However, no proliferative fibrous connective tissues and infiltrated inflammatory cells were observed in the adipocytes wrapped by the dermis in the IFD group, and the adipocyte morphology was normal (Figure 4).
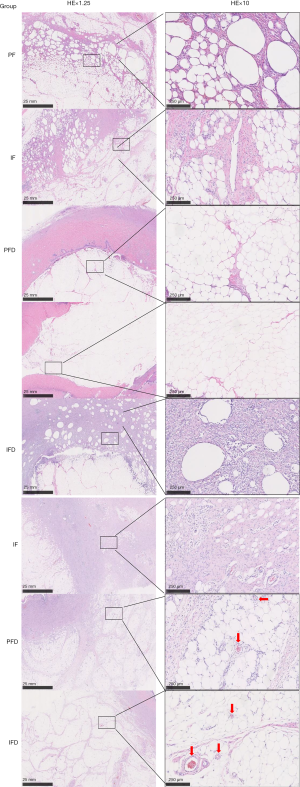
At the 30th day after implantation, adipocytes in the prostheses of PF and IF groups were almost completely absorbed and replaced by proliferative fibrous connective tissues, and a large number of infiltrated inflammatory cells were observed. No neovascularization was found in the adipose tissues. In the PFD group, a small amount of proliferative fibrous connective tissues and infiltrated inflammation cells were observed in adipocytes wrapped in the dermis. Meanwhile, neovascularization was observed in the adipose tissue and the morphology and structure of adipocyte were normal. However, a very small amount of proliferative fibrous connective tissues and infiltrated inflammatory cells were found in adipocytes wrapped by dermis in the IFD group. Neovascularization (red arrows) was also observed in these adipose tissues, and the adipocyte morphology was normal (Figure 4).
Discussion
Breast silicone gel prosthesis implantation is widely used for breast reconstruction domestically and overseas (13). After the breast is completely removed, breast silicone gel prosthesis is placed into the breast cavity or behind the pectoralis major muscle so as to achieve breast reconstruction. After implantation of the silica gel, the body will produce a foreign body reaction and local inflammation. It will then form a layer of membranous structures to encapsulate the prosthesis, known as the prosthesis capsule. In a few patients, this layer of capsule will become thick, hard, or even produce a compression prosthesis deformation and pain symptoms, known as a “capsular contracture” (14). In serious cases, it even induces local tissue canceration (15,16). Other complications included local flap necrosis, wound dehiscence, prosthesis rupture, prosthesis displacement, infection and so on (17).
The mold manufacturing process of the existing breast prosthesis is complex and generally designed only for patients who underwent entire breast gland excision, so specifications are very limited. However, the size and shape of the missing breast glands in early stage breast cancer patients after breast conservative surgery vary, thus no suitable prosthesis can be used. A crosslinked flexible elastic silicone rubber composition has been used to solve the problem of filling and shaping the tissue after partial breast gland loss (18), but these materials were chemical foreign bodies and not autologous tissue. After implantation, there are risks of allergy, rejection, carcinogenesis, rupture, aging, displacement and slippage, local tissue contracture around the prosthesis, limited in vivo time, and surgical removal. In addition, prosthesis materials are mainly imported and expensive. The authors made a conformal prosthesis from patients’ hair for breast reconstruction in patients with partial breast loss, but it is difficult to meet the needs of shaping due to the limited amount of hair in clinical practice (19).
Since Tuner and Bayram used autologous adipose tissue for facial injuries and breast reconstruction after total mastectomy, autologous adipose tissue transplantation had undergone numerous developments (20,21). Recently, with the standardization of liposuction surgery and the in-depth understanding of adipocytes and adipose-derived stem cells, autologous adipose tissue transplantation has once again become a hot research topic.
Autologous adipose tissue is considered as ideal fillers due to diverse sources, no foreign body reactions, and its soft characteristics (22,23). Current studies have confirmed that autologous adipose tissue transplantation has high applicative value in the repair of breast scar contractures, breast reconstruction, post-traumatic repair, and nipple reconstruction among other aspects (24,25). Nevertheless, the long-term effect of autologous adipose tissue transplantation is unstable, and imaging changes may occur after the operation. Due to the occurrence of large tissue defects, autologous adipose tissue transplantation is relatively limited in the application of total breast reconstruction (26). Therefore, there is much room for improvement in autologous adipose tissue transplantation for breast reconstruction.
Compared with other body parts, the breast has less blood supply and looser structure, so the rates of absorption and implantation-related complications are higher. Del Vecchio et al. showed that the implanted fat particles within 2 mm from microvessels could survive (27). Based on this problem, this study aimed to design inactivated autologous adipose tissues and dermal outer capsules to establish a breast prosthesis.
To our knowledge, the thickness of the dermis in the skin tissues is about 1–2 mm, and the dermis is mainly composed of fibroblasts, and the fiber and matrix they produce. It also contains blood vessels, lymphatic vessels, nerves, skin appendages, and other cellular components. The dermal matrix is made up of hydrophilic compounds composed of proteins and polysaccharides known as proteoglycans, such as hyaluronic acid, sulfur chondroitin, and so on. It is packed in the spaces between collagen fibers and fiber bundles and cells. The cutaneous immune reaction mainly occurs in the dermis. Mast cells, macrophages, and dendritic cells in the superficial dermis interact and regulate each other through the production of cytokines. This plays an important role in the activation, migration, proliferation, and differentiation of immune cells, the induction of immune response, inflammatory damage, and wound repair (28,29). When bacteria invade, inflammation and hypersensitivity can occur.
On one hand, it can block direct contact between autologous adipose tissues and other tissues, thus reducing the invasion of the peripheral inflammatory response and effectively delays the absorption of autologous adipose tissues. On the other hand, it can promote the regeneration of autologous adipose tissue vessels through the dermal immune response, thereby effectively improving the survival rate of autologous adipose tissues and maintaining the mammary shape after breast reconstruction. In addition, it can prevent the damage of the complement system to cells. Therefore, it is feasible to establish breast prosthesis with autologous adipose tissue with a dermal outer capsule.
In this study, autologous adipose tissues were heated at 56 °C for 30 minutes to inactivate the complement. This heat treatment also possibly inactivated any Mycoplasma in the serum.
The results of this study showed that fibrous connective tissue hyperplasia occurred in both autologous adipose tissues without a dermal outer capsule and inactivated autologous adipose tissues without a dermal outer capsule on the 14th day after implantation. On the 30th day after operation, the adipose tissues were almost completely absorbed and replaced by fibrous connective tissues, a large number of inflammatory cells had infiltrated, and no new angiogenesis was found in the adipose tissues. Autologous adipose tissues were almost fully absorbed, and as a result, the cosmetic effects were poor. Cigna et al. (30) did a satisfaction survey of breast reconstruction in patients receiving autologous adipose tissues and found that the average satisfaction of patients at the 6th month after operation was significantly lower than that at the 1st month, which was related to the high adipose tissue absorption rate and the difficulty in maintaining the repair effect.
In addition, this study also unexpectedly found that the morphology of autologous adipose tissues was clearer after heat inactivation in vitro. At the same time, it was also found that heat had a certain effect on the morphology of dermal tissue. Therefore, autologous adipose tissues were inactivated by heat while the dermis was not. The results of the in vivo experiment showed that dermal outer capsules plus autologous adipose tissues or inactivated autologous adipose tissues could significantly delay the absorption of autologous adipose tissues and maintain a good shape. In the PFD group, a small amount of connective tissue hyperplasia was observed in the adipose tissue on the 14th day, while more fibrous connective tissue hyperplasia and inflammatory cell infiltration were found in the adipose tissues, and new angiogenesis was also found in the adipose tissue on the 30th day. Thus, it is suggested that autologous adipose tissues could survive, but the whole prosthesis would not reach the soft degree of pure adipose tissues, which may be related to the existence of the dermis. In the IFD group, there was no obvious fibrous connective tissue hyperplasia in the adipose tissue at the 14th day, and only a small amount of fibrous connective tissue hyperplasia was observed in the adipose tissue at the 30th day. Meanwhile, the neovascularization significantly increased, and the adipose structure remained good.
From the above results, it can be seen that the prosthesis produced by inactivated autologous adipose tissues and dermal outer capsule has a better effect on the survival of adipose tissue and in reducing the absorption of adipose tissues after implantation. Its protective effects may be related to the following: (I) the dermis was used and the contact between dermis and peripheral skin is increased, which can induce the proliferation of subcutaneous tissues and blood vessels. The special properties of the dermis (such as proper thickness, hydrophilicity, immunoreactivity) and tension can provide a larger recipient area and a more favorable growth environment for the transplanted adipose tissues. (II) The use of inactivated autologous adipose tissue can not only sterilize the adipose tissues, but also reduce the damage of the complement system to the cells, promote angiogenesis, facilitate adipose tissue survival, and reduce adipose tissue absorption.
One of the major limitations of this study was that this study only assessed the cosmetic effects and histopathologic changes on the 30th day after implantation; thus, no information regarding late adipose tissue absorption and maintenance effects were gathered. Additionally, to evaluate the cosmetic effects better, we recommend the combination of various methods before analysis. Furthermore, the mechanism of inactivated autologous adipose tissues with a dermal outer capsule in vivo should also be explored further.
Conclusions
This experiment study indicated that the prosthesis produced by inactivated autologous subcutaneous adipose tissues with a dermal outer capsule may be an ideal prosthesis for breast reconstruction. Validation of this new prosthesis requires more experiments to assess the long-term cosmetic effects and histopathologic changes.
Acknowledgments
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-19-2844
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-19-2844
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-2844). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Zhejiang Cancer Hospital (No 2018-186). All animal tests were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health and efforts were conducted to minimize the number of animals used and any discomfort experienced.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Homsy A, Rüegg E, Montandon D, et al. Breast Reconstruction: A Century of Controversies and Progress. Ann Plast Surg 2018;80:457-63. [Crossref] [PubMed]
- Song WJ, Kang SG, Kim EK, et al. Current status of and trends in post-mastectomy breast reconstruction in Korea. Arch Plast Surg 2020;47:118-25. [Crossref] [PubMed]
- Kang CM, Shim JS. Volume change of pedicled latissimus dorsi muscle flap after partial breast reconstruction. J Reconstr Microsurg 2018;34:651-7. [Crossref] [PubMed]
- Fracol M, Grim M, Lanier ST, et al. Vertical skin paddle orientation for the latissimus dorsi flap in breast reconstruction: a modification to simultaneously correct inferior pole constriction and improve projection. Plast Reconstr Surg 2018;141:598-601. [Crossref] [PubMed]
- Lee BT, Agarwal JP, Ascherman JA, et al. Evidence-based clinical practice guideline: autologous breast reconstruction with DIEP or pedicled TRAM abdominal flap. Plast Reconstr Surg 2017;140:651e-664e. [Crossref] [PubMed]
- Cohen S, Sekigami Y, Schwartz T, et al. Lipofilling after breast conserving surgery: a comprehensive literature review investigating its oncologic safety. Gland Surg 2019;8:569-80. [Crossref] [PubMed]
- Chopan M, White JA, Sayadi LR, et al. Autogenous fat grafting to the breast and gluteal regions: safety profile including risks and complications. Plast Reconstr Surg 2019;143:1625-32. [Crossref] [PubMed]
- Herly M, Ørholt M, Larsen A, et al. Efficacy of breast reconstruction with fat grafting: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2018;71:1740-50. [Crossref] [PubMed]
- Darrach H, Kraenzlin F, Khavanin N, et al. The role of fat grafting in prepectoral breast reconstruction. Gland Surg 2019;8:61-6. [Crossref] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US);2011. Available online: http://nap.edu/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth
- U.S. Department of Health and Human Services. Guidance for industry and FDA staff: Saline, silicone gel, and alternative breast implants. Available online: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm071233.pdf
- Tyagi N, Sutton E, Hunt M, et al. Morphologic features of magnetic resonance imaging as a surrogate of capsular contracture in breast cancer patients with implant-based reconstructions. Int J Radiat Oncol Biol Phys 2017;97:411-9. [Crossref] [PubMed]
- Quesada AE, Medeiros LJ, Clemens MW, et al. Breast implant-associated anaplastic large cell lymphoma: a review. Mod Pathol 2019;32:166-88. [Crossref] [PubMed]
- Fitzal F, Turner SD, Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol 2019;9:190006. [Crossref] [PubMed]
- Ogita M, Nagura N, Kawamori J, et al. Risk factors for complications among breast cancer patients treated with post-mastectomy radiotherapy and immediate tissue-expander/permanent implant reconstruction: a retrospective cohort study. Breast Cancer 2018;25:167-75. [Crossref] [PubMed]
- Malice LF, Halley RJ. Self-forming partial breast prosthesis with encapsulated catalyst and a method of making the same: US, US6162250 [P]. 2000.
- Zou DH, Mao WM. Preparation methods of conformal prosthesis for partial loss of breast glands: CN, CN 103393481 A [P]. 2013.
- Turner A, Abu-Ghname A, Davis MJ, et al. Fat grafting in breast reconstruction. Semin Plast Surg 2020;34:17-23. [Crossref] [PubMed]
- Bayram Y, Sezgic M, Karakol P, et al. The use of autologous fat grafts in breast surgery: A literature review. Arch Plast Surg 2019;46:498-510. [Crossref] [PubMed]
- Raj S, Abu-Ghname A, Davis MJ, et al. Safety and Regulation of Fat Grafting. Semin Plast Surg 2020;34:59-64. [Crossref] [PubMed]
- Ruan QZ, Rinkinen JR, Doval AF, et al. Safety profiles of fat processing techniques in autologous fat transfer for breast reconstruction. Plast Reconstr Surg 2019;143:985-91. [Crossref] [PubMed]
- Howes BHL, Watson DI, Fosh B, et al. Efficacy of an external volume expansion device and autologous fat grafting for breast reconstruction following breast conserving surgery and total mastectomy: Small improvements in quality of life found in a prospective cohort study. J Plast Reconstr Aesthet Surg 2020;73:27-35. [Crossref] [PubMed]
- Krastev T, van Turnhout A, Vriens E, et al. Long-term follow-up of autologous fat transfer vs conventional breast reconstruction and association with cancer relapse in patients with breast cancer. JAMA Surg 2019;154:56-63. [Crossref] [PubMed]
- Qureshi AA, Odom EB, Parikh RP, et al. Patient-reported outcomes of aesthetics and satisfaction in immediate breast reconstruction after nipple-sparing mastectomy with implants and fat grafting. Aesthet Surg J 2017;37:999-1008. [Crossref] [PubMed]
- Del Vecchio D, Rohrich RJ. A classification of clinical fat grafting: different problems, different solutions. Plast Reconstr Surg 2012;130:511-22. [Crossref] [PubMed]
- Sobti N, Ji E, Brown RL, et al. Evaluation of acellular dermal matrix efficacy in prosthesis-based breast reconstruction. Plast Reconstr Surg 2018;141:541-9. [Crossref] [PubMed]
- Zingaretti N, Guameri GF, De Biasio F, et al. The use of meshed dermal autograft in breast reconstruction. Aesthetic Plast Surg 2018;42:1704-6. [Crossref] [PubMed]
- Cigna E, Ribuffo D, Sorvillo V, et al. Secondary lipofilling after breast reconstruction with implants. Eur Rev Med Pharmacol Sci 2012;16:1729-34. [PubMed]



