
B7-H7 is a prognostic biomarker in epithelial ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is one of the deadliest gynecologic cancer and the fifth leading cause of cancer death in women. Annually worldwide, 230,000 women are diagnosed and 150,000 die of EOC (1). According to global cancer statistics 2018, there were 295,414 new cases and 184,799 deaths of ovarian cancer worldwide. 90% of ovarian cancer is EOC (2). Because the ovarian tissue is located deep in the pelvic cavity, early symptoms of ovarian cancer are not obvious. To make matters worse, early effective screening method is very limited. About 75% patients already have advanced disease with metastasis upon diagnosis, only 20% of whom gain a survival duration of 5 years (3). Conventional management strategies for EOC include surgery and chemotherapy (4). However, in the past decades, the outcome of EOC patients has not significantly improved and the recurrence rate is still high. For patients with advanced disease, the traditional treatment is rather futile. Hence, development of novel therapeutics capable of improving patient survival is a top clinical priority in this population.
In recent researches of ovarian cancer, several co-stimulatory molecules such as B7-H3 and B7-H4 have been found to be prognostic biomarkers (5,6). PD-L1 also seems to be an independent prognostic factor for ovarian cancer (7). But it is still controversial whether PD-L1 is positive or negative factor (8).
One of the recent highlights in novel treatment options for cancer has been the targeting of immune checkpoint inhibitory molecules (9,10). Immune checkpoints are critically important in health and disease. They represent co-signaling pathways which are either co-stimulatory or co-inhibitory. There is accumulating evidence that the immune system plays an important role in the development and progression of EOC (11). Several checkpoint inhibitors have been widely researched in cancer therapy and some have been used in preclinical studies and clinical trials in patients with ovarian cancer such as anti-programmed cell death protein 1 (PD-1), anti-programmed death-ligand 1 (PD-L1) and anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies (12-14). It was reported that treatments blocking immune checkpoint molecules, PD-1 or PD-L1, have produced a beneficial and prolonged effect in a subgroup of these patients (13). However, the response rate of PD-1 inhibitor is rather low (only 5.9–20.0%) in ovarian cancer (15). This may be due to a lower PD-L1 expression level (around 40%) (16) and lower mutational burden in EOC cells when compared with other tumors (17). Furthermore, it seems that patients with EOC are relatively insensitive to single checkpoint therapy. Thus, there is an urgent need to discover additional targets for EOC immunotherapy to improve survival rate of ovarian cancer patients.
B7-H7 is a member of the B7 family. B7-H7 is also named as human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2), B7-H5 and B7y (18-20). It does not express in mice or rats (21). Human B7-H7 gene is located on chromosome 3q13.33 (18,21). The expression of B7-H7 has been reported in a majority of tumor specimens, including breast, lung, thyroid, melanoma, ovary, and pancreas tumors (20). B7-H7 was also widely found in many immune cells, such as antigen-presenting cells (APCs), monocytes, macrophages, dendritic cells and B cells, but not in T cells and natural killer cells (NK cells) (22,23). It can be up-regulated by inflammatory signals like lipopolysaccharide (LPS), interferon-γ (IFN-γ) and poly I: C (21). In tumor cells, the B7-H7 protein is located in both cytomembrane and cytoplasm (20,21,24). B7-H7 can regulate T cell function (22) and plays a significance role in tumor development and progression. According to the report by Janakiram et al., 50% of ovary cancer samples (4 of 8) expressed B7-H7 (20). However, the number of the cases is limited. The clinical significance of B7-H7 in EOC has not been fully investigated. Here, we analyzed B7-H7 expression by mIHC in a TMA of EOC, which involved 119 valid samples. We measured the expression level of B7-H7 in the array and evaluated the association between B7-H7 expression, clinical pathologic characteristics and prognosis.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-697).
Methods
Patients and tissue microarrays (TMAs)
We performed a retrospective study using a TMA (Catalog: HOvaC160Su01, Shanghai Outdo Biotech Co., Ltd., Shanghai, China). The TMA was constructed from 160 cases of surgically resected ovarian tissues (141 ovarian cancer tissues, 5 benign ovarian cancer tissues and 14 metastatic cancer tissues). The patients underwent surgery between February 2009 and February 2013. All patients’ follow-up periods were 5 to 9 years, and the follow-up period of the last case ended in March 2018. The follow-up endpoint was death. We excluded the benign ovarian cancer tissues, the metastatic cancer tissues and non-epithelial ovary caner tissues, and other cases were excluded due to incomplete clinical information and missing tissue samples during processing. Finally, a total of 119 cases were involved in the present work which were all EOC tissues. The recorded clinicopathological parameters included age, gender, survival status, overall survival (OS), disease-free survival (DFS), pathological character, as well as T, N, M, and FIGO stage. All the EOC cases had been classified according to the current dualistic model of epithelial ovarian carcinogenesis (type I and type II ovarian tumor) [2016] (25,26). The detailed parameters of the patients were shown in Table 1.
Table 1
| Characteristics | Value (%) |
|---|---|
| All | 119 (100.00) |
| Age | |
| <50 | 55 (46.22) |
| ≥50 | 64 (53.78) |
| Type | |
| I | 62 (52.10) |
| II | 57 (47.90) |
| FIGO stage | |
| I | 8 (6.72) |
| II | 28 (23.53) |
| III | 63 (52.94) |
| IV | 20 (16.81) |
| T stage | |
| T1 | 8 (6.72) |
| T2 | 28 (23.53) |
| T3 | 83 (69.75) |
| N stage | |
| N0 | 93 (78.15) |
| N1 | 26 (21.85) |
| M stage | |
| M0 | 99 (83.19) |
| M1 | 20 (16.81) |
| Survival | |
| Yes | 58 (48.74) |
| No | 61 (51.26) |
| Recurrence | |
| Yes | 96 (80.67) |
| No | 23 (19.33) |
All human tissues involved in this study were legitimate commercial products purchased from Shanghai Outdo Biotech CO., LTD. (http://www.superchip.com.cn/). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the company ethics committee (No. YB M-05-02) and informed consent was taken from all the patients. According to the company’s official website, the specimen bank had been approved by the IRB upon its establishment and the company has obtained all the informed consents before collecting all clinical samples.
Multiplex immunohistochemistry
For mIHC staining, an Opal 7-color Manual IHC Kit (NEL801001KT, PerkinElmer, USA) and VECTASHIELD® HardSet Antifade Mounting Medium (H-1400, Vector Labs, USA) were used. Anti-B7-H7 antibody (1:350, Abcam, ab214327) and anti-Cytokeratin (Pan) antibody (1:1, Maxim, Kit-0009) were simultaneously stained using a sequential staining protocol (27-29). First, the dilution and the proper antigen retrieval of the antibodies were optimized. The spectral library was built based on the single-stained slides. The TMA were baked at 63 °C for one hour and de-paraffinized by xylene and ethanol in automatic dyeing machine (LEICAST5020, LEICA). Then antigen retrieval was performed by microwave treatment (MWT) using antigen retrieval buffer (PH6.0) (AR6). After incubating with commercial 3% H2O2 for 10 minutes, the tissues were blocked in blocking buffer for another 10 minutes in moist chamber at room temperature. One primary antibody was incubated one hour at room temperature. The tissues were incubated with the secondary antibody and Opal working solution (1:100) (Opal520 for B7-H7 and Opal690 for CK) for 10 minutes at room temperature in sequence. The antigen retrieval (MWT) was performed to remove the primary/secondary antibody complexes with AR6 buffer and then repeated the staining cycles in series for the other primary antibody. After all antibodies were stained, we incubated the TMA with DAPI for 5 minutes then mounted TMA with Antifade Mounting medium.
Fluorescence signal quantification
The TMA was scanned using Vectra Polaris Automatic quantitative pathological imaging system (PerkinElmer, USA). Multispectral images were unmixed using spectral libraries built from images of single stained tissues for each reagent using the inform Advanced Image Analysis software (inForm 2.4.2; PerkinElmer, USA). The inForm software performed tissue segmentation (tumor compartment, stromal compartment and background compartment) based on the signal of pancytokeratin (27,30,31). A pathologist read the image and set the threshold value of B7-H7. The inForm software analyzed the images and performed the quantitation of B7-H7 immunostaining. Using the proportion of pixels at intensities meeting or exceeding a threshold value determined to label all positive antigen, the software calculated the percentage of positive stain. The final score was the percentage of positively stained cells.
Statistical analyses
According to the previous published studies B7-H7 positivity is around 50% in EOC, so we believe our sample size is large enough to generate reliable results. Analyses were performed using SPSS 22.0 statistical software and GraphPad Prism 7.0 software. Score difference between B7-H7 expression in different compartments were analyzed using Wilcoxon signed-rank test. The distribution of each categorical variable between B7-H7 high score and low score groups were assessed using chi square and Fisher’s exact test. Survival analysis was performed using Kaplan-Meier survival curves and Log-rank test. Cox regression analysis model, risk ratio (HR) and 95% confidence interval (CI) were used to assess the association between the B7-H7 expression and mortality risk. All statistical tests were two-sided. Statistical significance was considered at P<0.05.
Results
Expression of B7-H7 in EOC
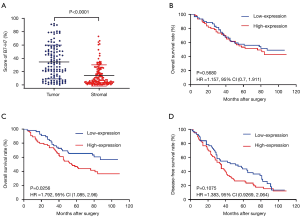
B7-H7 is broadly expressed in EOC. As shown in Figure 1, Cytokeratin (pan) positive staining was focal only in the cytomembrane and cytoplasm of cancer cells, which made it easy to accurately differentiate cancer cells from other cells according to the Cytokeratin (pan) positive staining. B7-H7 staining distributed in tumor and stromal compartments. B7-H7 was detected in cytomembrane and cytoplasm and was visible in tumor, stromal, and immune cells. B7-H7 expression was quantified as the percentage of positive cells on the TMA by mIHC. We obtained the quantifications of tumor compartment and stromal compartment separately in each core by segmenting the tissue. We found that all the samples expressed B7-H7. We compared the B7-H7 expression in tumor compartment to stromal compartment from the same tissue (Figure 2A). The median score was 31.5% in the tumor compartment and 7.35% in the stromal compartment. The score of B7-H7 expression was significantly higher in the tumor compartment than in stromal compartment (P<0.0001) by Wilcoxon signed-rank test.
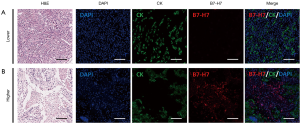
Association between B7-H7 expression and clinical characters
The association between B7-H7 expression and clinicopathological parameters in EOC was investigated by χ2 test and Fisher’s exact test.
The median score 31.51% was used as the cut-off value of high or low B7-H7 expression level in tumor compartment. In 119 cases, 49.6% (59/119) showed low B7-H7 expression in tumor compartment and 50.4% (60/119) showed high B7-H7 expression in tumor compartment. As listed in Table 2, significant positive correlation was found between age and B7-H7 expression in tumor compartment (χ2=6.127, P=0.013), whereas B7-H7 expression in tumor compartment was not significantly associated with type, FIGO stage, lymph node metastasis (N), distant metastasis (M), recurrence or survival in patients with EOC.
Table 2
| Clinicopathological characteristics | Cases | B7-H7 expression in tumor compartment | χ2 | P value | |
|---|---|---|---|---|---|
| Score ≤31.51% | Score >31.51% | ||||
| Age, years | 6.127 | 0.013 | |||
| <50 | 55 | 34 | 21 | ||
| ≥50 | 64 | 25 | 39 | ||
| Type | 0.074 | 0.786 | |||
| I | 62 | 30 | 32 | ||
| II | 57 | 29 | 28 | ||
| FIGO stage | 0.545 | 0.461 | |||
| I, II | 36 | 16 | 20 | ||
| III, IV | 83 | 43 | 40 | ||
| Lymphnodes metastasis (T) | 2.980 | 0.084 | |||
| Yes | 26 | 9 | 17 | ||
| No | 93 | 50 | 43 | ||
| Distance metastasis (M) | 0.002 | 0.967 | |||
| Yes | 20 | 10 | 10 | ||
| No | 99 | 49 | 50 | ||
| Recurrence | 0.035 | 0.851 | |||
| Yes | 96 | 48 | 48 | ||
| No | 23 | 11 | 12 | ||
| Survival status | 0.208 | 0.648 | |||
| Death | 61 | 29 | 32 | ||
| Survival | 58 | 30 | 28 | ||
The score 6.13% was used as the cut-off value of high or low B7-H7 expression level in stromal compartment. Cut-off values were determined using Cutoff Finder (http://molpath.charite.de/cutoff/) (32). In 119 cases, 46.2% (55/119) showed low B7-H7 expression in stromal compartment and 53.8% (64/119) showed high B7-H7 expression in stromal compartment. As listed in Table 3, significant correlation was found between B7-H7 expression in stromal compartment and FIGO Stage (χ2=4.605, P=0.045), lymph nodes metastasis (χ2=9.748, P=0.002), distance metastasis (χ2=4.354, P=0.049), and survival (χ2=5.190, P=0.028). B7-H7 expression in stromal compartment was not significantly associated with age, type, or recurrence in patients with EOC.
Table 3
| Clinicopathological characteristics | Cases | B7-H7 expression in stromal compartment | χ2 | P value | |
|---|---|---|---|---|---|
| Score ≤6.13% | Score >6.13% | ||||
| Age, years | 1.743 | 0.202 | |||
| <50 | 55 | 29 | 26 | ||
| ≥50 | 64 | 26 | 38 | ||
| Type | 3.869 | 0.066 | |||
| I | 62 | 34 | 28 | ||
| II | 57 | 21 | 36 | ||
| FIGO stage | 4.605 | 0.045 | |||
| I, II | 36 | 22 | 14 | ||
| III, IV | 83 | 33 | 50 | ||
| Lymph nodes metastasis (T) | 9.748 | 0.002 | |||
| Yes | 26 | 5 | 21 | ||
| No | 93 | 50 | 43 | ||
| Distance metastasis (M) | 4.354 | 0.049 | |||
| Yes | 20 | 5 | 15 | ||
| No | 99 | 50 | 49 | ||
| Recurrence | 1.218 | 0.353 | |||
| Yes | 96 | 42 | 54 | ||
| No | 23 | 13 | 10 | ||
| Survival status | 5.190 | 0.028 | |||
| Death | 61 | 22 | 39 | ||
| Survival | 58 | 33 | 25 | ||
Prognostic value of B7-H7 expressions in EOC patients
Survival data was available for all the patients in the TMA. Survival analysis was conducted using Kaplan-Meier survival curves and log-rank test. The B7-H7 expression level in tumor compartment was not significantly associated with OS in EOC [HR =1.157; 95% CI, (0.7, 1.911), P=0.5680] (Figure 2B). The Kaplan-Meier curve demonstrated that high expression of B7-H7 in stromal compartment predicted poor survival and high mortality rate in patient with EOC [HR =1.792; 95% CI, (1.085, 2.96), P=0.0256] (Figure 2C). However, B7-H7 expression in stromal compartment was not significantly associated with DFS in EOC [HR =1.383; 95% CI, (0.9269, 2.064), P=0.1075] (Figure 2D).
Subsequently, univariate and multivariate analyses of the Cox proportional hazard model on OS was carried out to determine the prognostic value of B7-H7 expression in stromal compartment with EOC and other clinicopathological variables (Table 4). In the univariate analysis, B7-H7 expression in stromal compartment showed significant correlation to the survival of patients with EOC [HR =1.796; 95% CI, (1.064, 3.032), P=0.028]. Meanwhile, type [HR =2.363; 95% CI, (1.406, 3.974), P=0.001], FIGO stage [HR =12.725; 95% CI, (3.981, 40.679), P<0.0001], lymph node metastasis [HR =5.5718; 95% CI, (3.355, 9.745), P<0.0001], and distant metastasis [HR =6.302; 95% CI, (3.556, 11.166), P<0.0001] were found to be associated with survival. The independent prognostic value was detected by multivariate analysis. The results indicated that FIGO stage [HR =8.508; 95% CI, (2.583, 28.029), P<0.0001] and distance metastasis [HR =2.292; 95% CI, (1.056, 4.649), P=0.036] were independent prognosis factors in the OS of patients with EOC. However, B7-H7 expression in the stromal compartment was not an independent prognostic factor in OS.
Table 4
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 0.103 | 0.544 | |||
| (≥50 vs. <50) | 1.535 (0. 918–2.567) | 1.181 (0.691–2.018) | |||
| Type | 0.001 | 0.635 | |||
| (II vs. I) | 2.363 (1.406–3.974) | 1.147 (0.650–2.026) | |||
| FIGO stage | <0.0001 | <0.0001 | |||
| (3-4:1-2) | 12.725 (3.981–40.679) | 8.508 (2.583–28.029) | |||
| Lymphnodes metastasis | <0.0001 | 0.067 | |||
| (N1 vs. N0) | 5.5718 (3.355–9.745) | 2.100 (0.948–4.649) | |||
| Distance metastasis | <0.0001 | 0.036 | |||
| (M1 vs. M0) | 6.302 (3.556–11.166) | 2.292 (1.056–4.649) | |||
| B7-H7 expression | 0.028 | 0.655 | |||
| (high vs. low) | 1.796 (1.064–3.032) | 0.871 (0.477~1.592) | |||
HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
To our knowledge, this is the first study using multiplexed quantitative immunofluorescence by segmenting tissues to examine the expression of B7-H7, its correlation with clinicopathologic character and prognostic significance in EOC. The mIHC combined with automatic quantitative pathological imaging system and inForm software analyses can increase the accuracy of the quantitation of each marker in the sample and even provide the per-cell quantitative data (31), which is an advantage compared with conventional IHC (33). The mIHC also maintains the morphological context of tissues so we can investigate the expression profiles of B7-H7 accurately on both immune cells and tumor cells, by segmenting tissues (based on Cytokeratin signal) (27). It is applicable to quantify B7-H7 separately because of the complexity of the B7-H7 expression location.
B7-H7 is a member of the B7 family of immune checkpoint molecules (18). It was revealed that B7-H7 has a 10–18% amino acid identity and 23–33% similarity to other B7 family members (21). But B7-H7 is not expressed in mice or rats (18,21). Previous studies measured the expression of B7-H7 in a variety of cancers and indicated variable expression in different histologic subgroups (20,34). Except cancer cells, B7-H7 has been found to be widely expressed in APCs and immune cells (21,22). B7-H7 binds to Transmembrane and immunoglobulin domain containing 2 (TMIGD2) and a potential second unknown receptor (21,22,35). TMIGD2 was found in naive T cells and NK cells, as well as endothelial cells and epithelial cells and have a role in angiogenesis (23,36). It is found that B7-H7 is a negative regulator of T cells because it could suppress proliferation of T cells and inhibit T-cell cytokine production (18). B7-H7 has been reported to have both co-inhibitory and co-stimulatory functions (21,22).
We found that there is a wide range of B7-H7 expression in EOC. Our data demonstrated that almost all of the EOC samples expressed B7-H7 in tumor and stromal compartments. Janakiram et al. (20) examined the expression of B7-H7 in human cancer tissue using IHC staining and showed that four out of eight ovarian cancer samples expressed B7-H7. Such inconsistency might have resulted from different experimental and quantitative methods, different sample sizes, and the fact that we only studied one specific type of ovarian cancer—EOC. We further demonstrated that B7-H7 was more expressed in the tumor compartment than in the stromal compartment. We plan to explore the effect of B7-H7 on ovarian cancer cell as well as immune cell function in our follow-up study. We will also try to investigate the mechanism of B7-H7 in EOC by upregulating or downregulating B7-H7 in ovarian cancer cell to observe its effect on cancer cell function. We also plan to co-culture ovarian cancer cells and T cells, and study the influence of B7-H7 on T cell activation and proliferation. The images in our research showed that B7-H7 staining located on cytomembrane and cytoplasm. Providing that B7-H7 is a transmembrane protein, this type of distribution is common and may be attributed to shuttling of the protein between the cytoplasm and the membrane (24). In order to reveal the association of B7-H7 and clinical characteristics and its prognosis significance in EOC, we analyzed B7-H7 expression in different compartment separately. Our data illustrated that in stromal compartment higher B7-H7 expression was significantly associated with advanced FIGO stage, distance metastasis, lymph node metastasis and poor OS. The Kaplan-Meier curve demonstrated that higher expression of B7-H7 in stromal compartment predicted poorer survival and higher mortality rate in EOC patient. In tumor compartment, higher B7-H7 expression was significantly associated with older age. However, there was only a marginal association between higher B7-H7 expression in tumor compartment and poor OS (P=0.568). These data suggested that B7-H7 expression was associated with more aggressive tumor behavior and lower survival rate in EOC patients. This finding was consistent with previous studies focusing on B7-H7 in a variety of other cancers (20,37-42). In a research of triple-negative breast cancer (TNBC), Janakiram et al. showed that overexpressed B7-H7 was associated with lymph node positivity, advanced stage of the disease, disease progress and metastasis (20). Zhu et al. (37) found that in colorectal carcinoma patients B7-H7 expression was significantly related to the depth of invasion, CD8+ T-cell infiltration status and high mortality rate, and that B7-H7 acted as an independent predictive factor in OS. Similarly, other studies demonstrated that B7-H7 could inhibit T cell function, and that higher expression of B7-H7 was correlated with poor survival in osteosarcoma, oral squamous cell carcinoma, intrahepatic cholangiocarcinoma and bladder cancer (39-42). The finding of Chen et al. demonstrated B7-H7 functioned as a co-inhibiting ligand in human clear cell renal cell carcinoma (38). They analyzed the effect of B7-H7 on cancer cell function and discovered that cell viability, migration and invasion ability were significantly inhibited upon knockdown of B7-H7 expression. They also found that B7-H7 expression level could be used as an independent risk factor for the prognostic prediction of clear cell renal cell carcinoma patients (38). All these indicated that B7-H7 is involved in the cancer immunosuppressive mechanisms and it is an inhibitory checkpoint. Since B7-H7 can suppress T cell functions, the up-regulation of B7-H7 expression on tumor cells and APCs provides a novel mechanism for tumor immune evasion. The different results shown in different compartments suggested that the B7-H7 expression in stromal compartment plays a more significant role to immunosuppressive in EOC microenvironment compared to tumor compartment.
Although our data suggest that B7-H7 is a promising line of inquiry in EOC, this study has certain limitations. One is that the sample size is relatively small. It may need an independent validated cohort for confirmation. Additional work is required to determine the functional significance of B7-H7 in ovarian cell lines and in the contest of B7-H7 function in ovarian tumor immune system.
Conclusions
In conclusion, our findings demonstrate that B7-H7 expression was significantly associated with tumor progression and prognosis in patients with EOC. High expression of B7-H7 in EOC stromal compartment predicted a low survival rate. As a result, B7-H7 may serve as a critical immune checkpoint in EOC and as a potential therapeutic target for EOC patients.
Acknowledgments
We appreciate the help from Andrew Fesler with the language of the manuscript.
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-697
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-697
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-697
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-697). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the company ethics committee (No. YB M-05-02) and informed consent was taken from all the patients. According to the company’s official website, the specimen bank had been approved by the IRB upon its establishment and the company has obtained all the informed consents before collecting all clinical samples.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Drakes ML, Mehrotra S, Aldulescu M, et al. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J Ovarian Res 2018;11:43. [Crossref] [PubMed]
- Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet 2019;393:1240-53. [Crossref] [PubMed]
- Zhang J, Liu L, Han S, et al. B7-H3 is related to tumor progression in ovarian cancer. Oncol Rep 2017;38:2426-34. [Crossref] [PubMed]
- Ye Y, Wang JJ, Li SL, et al. Does B7-H4 expression correlate with clinicopathologic characteristics and survival in ovarian cancer?: A systematic review and PRISMA-compliant meta-analysis. Medicine (Baltimore) 2018;97:e11821. [Crossref] [PubMed]
- Schmoeckel E, Hofmann S, Fromberger D, et al. Comprehensive analysis of PD-L1 expression, HER2 amplification, ALK/EML4 fusion, and mismatch repair deficiency as putative predictive and prognostic factors in ovarian carcinoma. Virchows Arch 2019;474:599-608. [Crossref] [PubMed]
- Wang Q, Lou W, Di W, et al. Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol 2017;52:7-14. [Crossref] [PubMed]
- Janakiram M, Pareek V, Cheng H, et al. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunotherapy 2016;8:809-19. [Crossref] [PubMed]
- Sankin A, Narasimhulu D, John P, et al. The expanding repertoire of targets for immune checkpoint inhibition in bladder cancer: What lies beneath the tip of the iceberg, PD-L1. Urol Oncol 2018;36:459-68. [Crossref] [PubMed]
- Hardwick N, Frankel PH, Cristea M. New Approaches for Immune Directed Treatment for Ovarian Cancer. Curr Treat Options Oncol 2016;17:14. [Crossref] [PubMed]
- Pujade-Lauraine E, Fujiwara K, Dychter SS, et al. Avelumab (anti-PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 Phase III study design. Future Oncol 2018;14:2103-13. [Crossref] [PubMed]
- Pietzner K, Nasser S, Alavi S, et al. Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol 2018;29:e93. [Crossref] [PubMed]
- Hamanishi J, Mandai M, Konishi I. Immune checkpoint inhibition in ovarian cancer. Int Immunol 2016;28:339-48. [Crossref] [PubMed]
- Ring KL, Pakish J, Jazaeri AA. Immune Checkpoint Inhibitors in the Treatment of Gynecologic Malignancies. Cancer J 2016;22:101-7. [Crossref] [PubMed]
- Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014;23:2965-70. [Crossref] [PubMed]
- Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [Crossref] [PubMed]
- Flajnik MF, Tlapakova T, Criscitiello MF, et al. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7's historical relationship with the MHC. Immunogenetics 2012;64:571-90. [Crossref] [PubMed]
- Mager DL, Hunter DG, Schertzer M, et al. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3). Genomics 1999;59:255-63. [Crossref] [PubMed]
- Janakiram M, Chinai JM, Fineberg S, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res 2015;21:2359-66. [Crossref] [PubMed]
- Zhao R, Chinai JM, Buhl S, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A 2013;110:9879-84. [Crossref] [PubMed]
- Zhu Y, Yao S, Iliopoulou BP, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun 2013;4:2043. [Crossref] [PubMed]
- Janakiram M, Chinai JM, Zhao A, et al. HHLA2 and TMIGD2: new immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology 2015;4:e1026534. [Crossref] [PubMed]
- Janakiram M, Shah UA, Liu W, et al. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev 2017;276:26-39. [Crossref] [PubMed]
- Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol 2016;186:733-47. [Crossref] [PubMed]
- Rojas V, Hirshfield KM, Ganesan S, et al. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int J Mol Sci 2016;17:2113. [Crossref] [PubMed]
- Gorris MAJ, Halilovic A, Rabold K, et al. Eight-Color Multiplex Immunohistochemistry for Simultaneous Detection of Multiple Immune Checkpoint Molecules within the Tumor Microenvironment. J Immunol 2018;200:347-54. [Crossref] [PubMed]
- Kalra J, Baker J. Multiplex Immunohistochemistry for Mapping the Tumor Microenvironment. Methods Mol Biol 2017;1554:237-51. [Crossref] [PubMed]
- Carstens JL, Correa de Sampaio P, Yang D, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun 2017;8:15095. [Crossref] [PubMed]
- Schalper KA, Carvajal-Hausdorf D, McLaughlin J, et al. Differential Expression and Significance of PD-L1, IDO-1, and B7-H4 in Human Lung Cancer. Clin Cancer Res 2017;23:370-8. [Crossref] [PubMed]
- Mansfield JR. Cellular context in epigenetics: quantitative multicolor imaging and automated per-cell analysis of miRNAs and their putative targets. Methods 2010;52:271-80. [Crossref] [PubMed]
- Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012;7:e51862. [Crossref] [PubMed]
- Zhang W, Hubbard A, Jones T, et al. Fully automated 5-plex fluorescent immunohistochemistry with tyramide signal amplification and same species antibodies. Lab Invest 2017;97:873-85. [Crossref] [PubMed]
- Cheng H, Janakiram M, Borczuk A, et al. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin Cancer Res 2017;23:825-32. [Crossref] [PubMed]
- Xiao Y, Freeman GJ A. New B7:CD28 Family Checkpoint Target for Cancer Immunotherapy: HHLA2. Clin Cancer Res 2015;21:2201-3. [Crossref] [PubMed]
- Rahimi N, Rezazadeh K, Mahoney JE, et al. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell 2012;23:1646-56. [Crossref] [PubMed]
- Zhu Z, Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther 2018;11:1563-70. [Crossref] [PubMed]
- Chen L, Zhu D, Feng J, et al. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int 2019;19:101. [Crossref] [PubMed]
- Lin G, Ye H, Wang J, et al. Immune Checkpoint Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 is Upregulated and Independently Predicts Unfavorable Prognosis in Bladder Urothelial Carcinoma. Nephron 2019;141:256-64. [Crossref] [PubMed]
- Xiao Y, Li H, Yang LL, et al. The Expression Patterns and Associated Clinical Parameters of Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 and Transmembrane and Immunoglobulin Domain Containing 2 in Oral Squamous Cell Carcinoma. Dis Markers 2019;2019:5421985. [Crossref] [PubMed]
- Jing CY, Fu YP, Yi Y, et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer 2019;7:77. [Crossref] [PubMed]
- Koirala P, Roth ME, Gill J, et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep 2016;6:31154. [Crossref] [PubMed]


