
Impact of preoperative magnetic resonance imaging in breast cancer patients candidates for an intraoperative partial breast irradiation
Introduction
Breast conserving surgery (BCS) followed by whole breast irradiation (WBI) is recognized as the standard of care for early breast cancer (BC). Based upon local failure patterns, partial breast irradiation (PBI) (targeting the tumor bed) has been considered for early BC patients, in order to shorten the treatment time, reduce the radio-induced toxicity, therefore improving quality of life. However, treatment’s de-escalation remains to be proven equivalent in terms of local control to the gold standard WBI, and PBI is currently still debated due to lack of results of randomized trials with sufficient follow-up. Nonetheless, PBI is increasingly used due to its convenience for patients and its advantages related to radiation facilities access. If this shortened treatment would become standard, only highly selected patients would benefit from this kind of treatment. Currently, many questions regarding appropriate patient selection criteria still exist, despite the fact that both the American and European societies of Radiology and Oncology (ASTRO, GEC-ESTRO) provided recommendations for patients treated off clinical trials, based on tumor- and patient characteristics, recommendations that slightly differ between each society (1,2). These patient selection criteria are likely the cornerstone of PBI’s success. The challenge is to avoid this “lightest” treatment for patients with a high ipsilateral recurrence risk. Magnetic resonance imaging (MRI) is a highly sensitive exam in detecting clinically occult BC, not highly specific, and has never been proven improve local recurrence rates (LRR) when used as part of preoperative staging in patients treated with BCS followed by WBI (3,4). On the contrary, the routine use of breast MRI for preoperative staging is not recommended since it may modify the surgery type thus increasing the mastectomy rate (3). In patients planned for a PBI, conditions are different because an occult cluster, remote in the ipsilateral breast will be ignored by the partial breast treatment and may result in an increase of LRR. That is why the value of breast MRI for preoperative staging in this setting deserves assessment. ASTRO Task Force does not support the routine use of preoperative breast MRI in patient selection for PBI. Neither in the TARGIT-A trial, nor in the ELIOT trial, preoperative breast MRI was required, and was just performed at the discretion of the physician. Intraoperative radiotherapy (IORT) using low-energy X-rays of 50 kV is one of the methods of PBI used in France, currently under investigation in clinical trials and used sometimes off protocol. Patient selection for PBI varies according to the clinical trial, but is usually based on recommendations close to those provided by both ASTRO and GEC-ESTRO. This retrospective study was conducted in order to assess the impact of preoperative MRI on patient eligibility for PBI, and to determine the rate of unsuspected ipsilateral second cancer on mammography, in a highly selected population. It was also to assess the number of additional exams prompted by MRI abnormalities and MRI’s relevance, in order to avoid useless exams for those patients at supposed very low risk of second ipsilateral cancer.
Methods
Patients
From March 2012 through February 2014, BC patients meeting the Inca’s criteria for PBI were offered the possibility of a shortened treatment through IORT, either in a prospective trial or off protocol (all patients gave written or oral consent, registered in the medical chart). Data were monitored prospectively. The eligibility criteria, based on physical examination, mammography and ultrasound, and a biopsy pathological exam, were as follows: menopaused woman, 55 years or older with T1, N0, hormonal-receptor-positive and HER2-negative, invasive non-lobular epithelioma, without extensive intraductal component (defined as more than 25% of ductal component on biopsy), without fast-growing tumor, without lymphovascular invasion (LVI), without criteria for adjuvant chemotherapy. Preoperative assessment included a tumor biopsy for pathological assessment that had to provide as many information as possible for selecting patients meeting the required criteria.
Breast MRI
Breast MRI was performed on a 1.5-T GE MR scanner HDxT (General Electric, Milwaukee, WI) using a dedicated phased array bilateral breast coil. Patients were imaged in the prone position. First, morphologic sequences were acquired using an axial echo gradient 3D T1-weighted sequence (TR/TE: 8/4; flip angle: 15°; no gap; field of view: 35 cm; matrix: 512/320; number of excitations: 0.7; scanning time: 90 sec) and a 2D T2-weighted sequence (TR/TE: 3220/85; flip angle: 90°; no gap; field of view: 35 cm; matrix: 352/352; number of excitations: 2; scanning time: 4 min 40 sec). Then, an axial 3D dynamic contrast-enhanced T1-weighted fat-saturated gradient-echo sequences was acquired (TR/TE: 5.4/2.6; flip angle: 15°; slice thickness: 2 mm; no gap; field of view: 35 cm; matrix: 416/416; number of excitations: 0.7; scanning time: 7 min 25 sec). These sequences were acquired before and six times after bolus injection of gadolinium chelate (Dotarem, Guerbet, France) (0.1 mmol·kg-1 body weight) given via a power injector (Mallinckrodt, St Louis, MO, USA). All of the MR images were reviewed on the ADW console (General Electric). A board certified radiologist with 10 years of experience in breast MRI, reviewed all the breast MRI. The radiologist first interpreted the MR images and looked for the presence or absence of additional lesions. An additional lesion was defined as a lesion separate from the index tumor undetected by conventional methods (mammography and ultrasonography) but detected by sequentially performed contrast-enhanced breast MRI. If additional lesions were detected on MRI, the reader classified the lesions based on the second edition of BI-RADS MR lexicon and assigned a BI-RADS category.
Treatments
IORT was performed for all patients in a single surgical procedure. The sentinel node biopsy as well as instantaneous pathological analysis were performed before IORT which was maintained if the results were negative. Surgical excision was performed from the skin up to the pectoral fascia in every case. Margin status was assessed intraoperatively through a fast frozen section analysis to ensure clear margins before the IORT procedure; if this margin was positive or close, a re-resection was performed before IORT. The skin was spared from radiation dose by properly applied water-coated gauze and was maintained at more than 10 mm from the applicator surface. A radiation shield material was applied on the pectoral muscle to avoid a high single radiation dose in the ribs.
Data collection and procedures
For this retrospective analysis, we reviewed all MRI reports from patients scheduled for PBI (having had a preoperative MRI), and searched for abnormalities not detected by mammograms or ultrasound. We recorded the location of the occult foci (same quadrant or remote in the breast), their distance relative to the index lesion and the results of pathologic evaluation when subsequent biopsy was performed. The supplemental foci classified as ACR 3-4, prompted a second-look focused ultrasound which, either has invalidated a supplemental lesion, or confirmed a suspicious lesion which therefore has led to a pathologic assessment through a micro-biopsy. The supplemental foci, classified ACR5, were systematically submitted to biopsy. Multifocal disease was defined as one (or more) additional lesion, biopsy-proven, in the same quadrant, whereas multicentric disease was defined as one (or more) additional lesion, biopsy-proven, at more than 4 cm from the index lesion or in another quadrant. Mammographic breast density was described for all patients using the BIRADS lexicon of the American College of Radiology. The breast density was divided in four categories: a breast entirely fatty, was defined as a “density 1”; Breast with scattered fibroglandular densities (approximately 25-50% glandular): “density 2”; Breast with scattered fibroglandular densities (approximately 51-75% glandular): “density 3” and finally, an extremely dense breast tissue more than 75% glandular was defined as a “density 4”.
Statistical analysis
Patient’s treatments and tumors’ characteristics were summarized using means, standard deviations, medians and ranges for quantitative variables and counts and percentages for categorical variables. The incidence of additional ipsilateral BC in the present study population was defined as the primary evaluation criterion. The proportion of patients presenting an ipsilateral additional BC was estimated with 95% confidence intervals using an exact binomial method (5). Statistical analysis was performed using SAS v9.3 sofware (SAS institute Inc. Cary, NC, USA). This retrospective study was approved by our institutional review board.
Results
Between March 2012 and February 2014, 179 early BC patients meeting the required criteria were planned for PBI. Seventy nine percent of them (141/179) underwent an MRI as part of preoperative staging, and constituted the study population. Thirty eight patients did not performed a pre-operative MRI, mainly due to surgeon’s preference (27/38 patients) or due to MRI contraindication (5 patients), or patient refusal (4 patients) or other cause (2 patients).
Patient characteristics
Clinical and pathological characteristics of the 141 patients who underwent a preoperative MRI are detailed in Table 1. Median age was 67 years (range, 55-90 years). Three fourth of patients were classified as T0N0 and median pathologic size was 12 mm. The tumor grade was correctly assessed on biopsy. LVI was missed by biopsy in 16% of patients and lymph-node involvement has also been ignored by clinical exam and imaging in 16% of patients.
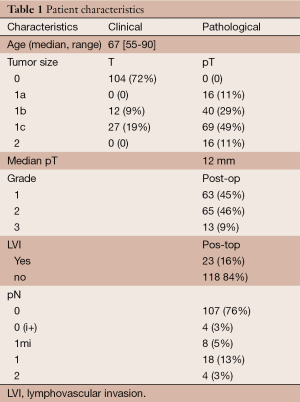
Full table
Breast MRI new abnormalities and second cancer rate
The preoperative breast MRI has identified ACR3-4 new abnormalities in 31% of patients (44/141), either in ipsilateral or contralateral breast or both (Table 2). Overall, subsequently to focused ultrasound, a biopsy has been required for 29/141 patients (21%). Suspicious ipsilateral lesions were found in 33/141 patients (23%), distributed as shown in Table 2, and were invalidated by focused ultrasound in 12 patients; thus the ipsilateral MRI abnormalities have prompted a biopsy for 21/141 patients (15%). A second ipsilateral cancer was confirmed in one fourth of biopsied patients, and led to the IORT-PBI cancellation in 4% of patients having had a preoperative MRI. Suspicious contralateral lesions were found in 19/141 patients (13%). Focused ultrasound have invalidated four of them, having required a biopsy for 15 patients (11%; some of them have already been accounted due to bilateral additional abnormalities). A contra-lateral BC was found in 6/141 patients (4.3%; 95% CI: 1.5-9.0), and IORT-PBI was cancelled only for the patient presenting both an ipsilateral and contralateral second cancer. In other words, a contralateral synchronous BC has not precluded the planned IORT treatment, which has sometimes been performed for the two sides.
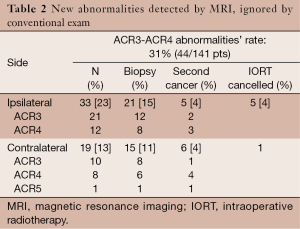
Full table
Additional ipsilateral cancer location
The distance between the external edges of the two ipsilateral lesions was found to be 45 to 90 mm (mean 50 mm), with two bifocal lesions, in the same quadrant, becoming bi-centric due to the distance of more than 40 mm between the two lesions, two bi-centric lesions (in two different quadrants) and one multicentric lesion. Breast density for these patients was two in most of cases.
Discussion
BCS followed by WBI is recognized as the standard of care for early BC. WBI consists of 50 Gy/2 Gy per fraction/25 fractions/5 weeks in the whole breast followed by a 10-16 Gy boost to the tumor bed. This regimen has been proven being able to allow a 5- and 10-year LRR of 4% and 8% respectively for unselected patients more than 50 years old (6). In selected patients, >50 years old with favorable tumor characteristics, lower LRR could be expected. Any change in radiotherapy schedule must guarantee the same LRR without increasing toxicity. The rationale for PBI is based upon the fact that although more than half of specimens of mastectomies undertaken for small BC harbor occult cancer foci remote from the index lesion (7,8), at least three fourth of LR are true recurrences (occurring in the initial tumor bed) (9). This fact has been observed in patients treated with BCS followed by WBI, as well as in patients treated with BCS alone (without WBI) (9). Nonetheless, BC recurrence outside the index quadrant appears to be significantly lowered by adjuvant WBI, from 1.5-3.5% to 0.5-1% (9-11). In other words, the role of WBI is to reduce the recurrence risk in the tumor bed, as well as remote in the breast. It could be argue that the risk of recurrence outside of the initial tumor bed is so low that slightly increasing this risk is acceptable when, on the other side 3 to 5 weeks of radiation treatment is avoided. But this 1.5% to 3.5% recurrence risk, remote from the index lesion, observed when adjuvant WBI is omitted, is not so far from the 4% of occult second cancer in the ipsilateral breast revealed by breast MRI, and can therefore be avoided by a preoperative MRI. And it has also been suggested that patients are willing to accept treatments inconvenience for an expected 1% benefit on recurrence risk (12). Noteworthy, in this situation, it’s not treatment convenience which is discussed but only an additional exam to lower the recurrence risk. The use of preoperative MRI has been accused to unnecessarily increase the mastectomy rate. This is actually true only when a histologic confirmation is not done. The TARGIT-A, a prospective randomized trial, have reported the non-inferiority of intraoperative PBI compared to WBI in terms of LR (5-y LR: 2.1% in the IORT arm vs. 1.1% in the WBI arm; P=0.31, for the subgroup of 2298 patients treated with IORT concurrently with lumpectomy or lumpectomy followed by WBI), with a median follow-up of 2 years and 5 months for the whole cohort (13). Patients included in this trial had to be ≥45 years old, T2-3 unifocal invasive ductal BC (lobular carcinoma was not permitted), suitable for breast-conserving surgery, N0-1 nodal status. Preoperative breast MRI was not mandatory. This non-inferiority study was powered to detect a 2.5% absolute difference in local recurrence between the two arms. The authors concluded that IORT concurrent with lumpectomy within a risk-adapted approach should be considered as an option for eligible patients with BC carefully selected as per the TARGIT-A trial protocol, as an alternative to postoperative WBI. It seems that the recurrence rate remote from the index lesion is until then the same in the two arms, with a relatively short follow-up, not long enough to definitely conclude. The results of the ELIOT trial have recently been reported (14). This randomized controlled trial has included 1,305 patients randomly assigned to IORT or WBI. Patient selection was less stringent than in TARGIT-A trial, (more advanced disease). The authors have detailed the sites of recurrence and reported an increased ipsilateral recurrence rate both in the tumor bed and remote from the index lesion. Patients receiving WBI did not experienced any recurrence outside the tumor bed at 5 years, whereas patients in the IORT group had a 1.9% recurrence rate remote from the index lesion (P=0.0001). Several studies have examined the potential of preoperative breast MRI to improve patient’s selection for PBI (15-20). The rate of ipsilateral additional BC was reported ranging from 2.8% to 10%, depending of the robustness of patient selection and correlated with known prognostic factors for local recurrence such as tumor size, age less than 50 years, LVI and HER2-positive tumors (19). Our data show that in highly selected patients considered as candidates for IORT, 4% presented with a bifocal tumor, which could result in a 4% recurrence rate in the follow-up (excluding true LR), higher than that expected. These results compare favorably with those from other series having tested the role of preoperative MRI in patient selection for PBI. MRI seems therefore an interesting exam in selecting candidates for PBI and should be performed systematically despite its high level of false positive, in order to not sentencing PBI for the wrong reasons. This practice has been approved by the EUSOMA working group with a high level (B) of recommendation (21). The limitations of this study include its retrospective nature, the small study population and small number of events precluding an analysis of clinical or pathological factors correlated with the presence of additional cancer. We would be for example interested in defining or exclusion of the risk depending on the breast density. Finally, the real significance of these occult additional lesions is not known and it is not clear if these lesions could be indolent lesions. That is why comparison of patients treated with PBI with or without the routine use of preoperative MRI would answer this question.
Conclusions
The use of preoperative MRI in patient staging leads to diagnosis of an ipsilateral second BC in 4% of cases, which appears substantial in a highly selected population. We therefore support the routine use of this exam for the staging of patient candidate for a PBI.
Acknowledgments
We thank all the authors for their contribution in the recruitment, treatment, follow-up of the patients and for having participated in the elaboration and writing of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frank A. Giordano, Pedro Carlos Lara and Frederik Wenz) for the series “Intraoperative Radiotherapy II” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.04.04). The series “Intraoperative Radiotherapy II” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by our institutional review board. All patients gave written or oral consent, registered in the medical chart.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [PubMed]
- Polgár C, Van Limbergen E, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264-73. [PubMed]
- Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin 2009;59:290-302. [PubMed]
- Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol 2014;32:392-401. [PubMed]
- Feller W. An introduction to probability theory and its applications Vol1, 3rd edition. New York: Wiley, 1968.
- Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 2007;25:3259-65. [PubMed]
- Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985;56:979-90. [PubMed]
- Vaidya JS, Vyas JJ, Chinoy RF, et al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820-4. [PubMed]
- Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol 2001;12:997-1003. [PubMed]
- Clark RM, McCulloch PB, Levine MN, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst 1992;84:683-9. [PubMed]
- Liljegren G, Holmberg L, Adami HO, et al. Sector resection with or without postoperative radiotherapy for stage I breast cancer: five-year results of a randomized trial. Uppsala-Orebro Breast Cancer Study Group. J Natl Cancer Inst 1994;86:717-22. [PubMed]
- Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol 1998;16:515-21. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [PubMed]
- Al-Hallaq HA, Mell LK, Bradley JA, et al. Magnetic resonance imaging identifies multifocal and multicentric disease in breast cancer patients who are eligible for partial breast irradiation. Cancer 2008;113:2408-14. [PubMed]
- Godinez J, Gombos EC, Chikarmane SA, et al. Breast MRI in the evaluation of eligibility for accelerated partial breast irradiation. AJR Am J Roentgenol 2008;191:272-7. [PubMed]
- Tendulkar RD, Chellman-Jeffers M, Rybicki LA, et al. Preoperative breast magnetic resonance imaging in early breast cancer: implications for partial breast irradiation. Cancer 2009;115:1621-30. [PubMed]
- Horst KC, Fero KE, Ikeda DM, et al. Defining an optimal role for breast magnetic resonance imaging when evaluating patients otherwise eligible for accelerated partial breast irradiation. Radiother Oncol 2013;108:220-5. [PubMed]
- Dorn PL, Al-Hallaq HA, Haq F, et al. A prospective study of the utility of magnetic resonance imaging in determining candidacy for partial breast irradiation. Int J Radiat Oncol Biol Phys 2013;85:615-22. [PubMed]
- Kühr M, Wolfgarten M, Stölzle M, et al. Potential impact of preoperative magnetic resonance imaging of the breast on patient selection for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2011;81:e541-6. [PubMed]
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010;46:1296-316. [PubMed]


