
Wound response after intraoperative radiotherapy
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide and the second leading cause of cancer-related death. More than 1-2 million cases are diagnosed every year, affecting 10-12% of the female population and accounting for 500,000 deaths per year (1). In the last two decades, mortality rates have generally remained stable or slightly decreased. Declines in BC mortality have been attributed to both novel treatment strategies and early detection due to the implementation of screening/prevention programs (2).
BC is not a single disease: it is instead a collection of breast diseases that are heterogeneous in terms of histology, genetic and genomic variations, therapeutic response and patient outcomes (3). From a clinical point of view, BC can be subdivided into three major subtypes: tumors expressing estrogen receptors (ERs) and/or progesterone receptors (PRs), tumors expressing amplified form of human epidermal receptor 2 (HER2-amplified) and tumors commonly referred to as triple-negative BC (TNBC), due to lack of or low positivity for ER, PR and HER2 (4). These markers together with other clinical parameters (age, node status, tumor size, histological grade) are routinely used in the clinic to stratify patients for prognostic predictions and treatment selection. However, the complexity of BC disease is not entirely reflected by the parameters described. More recent studies have provided new ways of classification of BC patients, based on variations in their gene expression profiles that correlated with prognosis (5-8), establishing five BC intrinsic subtypes (Luminal A, Luminal B, HER2-enriched, Claudin-low, Basal-like) and a normal breast-like group.
Importantly, these subtypes have been shown to be clinically meaningful and can divide patients into groups with distinct tumor histotypes and distinct outcomes. However, up to now the expression of the ER, PR, and HER2 receptors that are routinely and easily evaluated in any pathology, are still the parameters used by oncologists to guide therapy decisions. For BC patients, several treatment options are currently available in neoadjuvant and adjuvant settings. These include hormone therapies, targeted therapies, radiotherapy (RT) and various chemotherapy regimens.
Local recurrence in early breast cancer (EBC)
The systemic use of widespread mammographic screening has contributed to a stage shift for newly diagnosed disease, increasing the percentage of EBC at diagnosis. In women with EBC all detectable cancer is restricted to the breast and, in women with node-positive disease, to the local lymph nodes. Breast conserving therapy, including primary tumor excision, axillary node dissection (determined in advance or decided following sentinel node sampling) and external RT, is considered standard of care for management of women with EBC (9). For EBC patients the appearance of local relapse (LR, defined as the reappearance of malignant disease in the ipsilateral breast) represents a common event that may influence the prognosis. Several studies have shown that the presence of local recurrence is the strongest independent predictor of distant relapse and confers three-fold to four-fold increased risk of progression (10). Importantly, it was demonstrated that local recurrence formation is causally related to distant relapse, indicating that it is a determinant and not simply an indicator of augmented risk (11). Disease relapse occurs in one out of five BC patients and represents the principal cause of BC-related deaths (12). The relative risk of distant metastases for patients developing LR in comparison with patients without LR is considerable and, in fact, patients who develop LR present a substantially worse overall survival (13-15). The importance to restrain local recurrences in BC patients has been recently highlighted in a overview that conclusively showed that treatments substantially improving local control have better effects on long-term survival, representing one life saved for every four loco-regional recurrences prevented (16).
The effects of external RT on local as well as distant recurrence formation and on long-term overall survival of BC patients were recently extensively analyzed. A recent meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has demonstrated that local radiation treatment to either the breast after breast-conservation surgery or the chest wall after mastectomy induced an overall survival benefit at 15 years (16). In particular, RT reduces the recurrence rate by the half and the death rate by about one sixth in patients that have undergone breast conserving surgery. Results of the EBCTCG overview have thus reinforced the link between local control and mortality, emphasizing the importance of achieving the best loco-regional treatment for this kind of patients.
Local recurrence in early breast cancer (EBC) patients and the wound response
Clinical and experimental data suggest that the perturbation induced by surgery itself and the subsequent wound healing process may result in stimulation of residual disease (17-19). From a clinical point of view, 90% of local recurrences occur at or close to the same quadrant of the primary cancer, despite multifocality and multicentricity are very common in BC (20). Three-dimensional analysis of specimens from mastectomy show that 63% of breasts harbor occult cancer foci, with 80% of these situated remote from the index quadrant. However, the cancers in other quadrants of the breast appear to remain dormant for many years and have a low risk of developing clinically relevant tumors (20). The results of a randomized clinical trial comparing the effects of mastectomy with quadrantectomy showed that early relapses were more frequent in the mastectomy than in the quadrantectomy group. This difference, which disappeared later in time during follow-up, is consistent with an initial acceleration of metastatic burden after more aggressive surgery (14). These clinical observations support the idea that local disease develops from growth of residual BC cell in peritumoral tissue, in response to the inflammatory and wound-healing stimuli elicited by surgery. Experimental data in animal models show that the surgical trauma enhances the proliferation of metastatic foci, supporting the hypothesis that surgery should be considered a major perturbing factor for metastasis growth (21,22). Similarly to what observed in animal models, also in human BC some evidences highlight that surgical removal of the primary tumor may induce changes in the growth kinetics of micro-metastasis (18).
Wound healing and cancer progression have striking similarities, including inflammation, the growth of new blood vessels (angiogenesis), the rearrangement of the molecular matrix around the cells, and changes in how cells attach to each other, leading to the definition of tumors as wounds that do not heal (23-25). Moreover, the molecular programs in normal wound healing and those in tumor progression and metastasis were found to be similar. The correlation between wound response and cancer progression was also supported by the analysis of gene expression profile of normal tissue adjacent to cancer that evidences the activation of a “wound response signature” able to promote cancer progression (26). The activation of this “wound response signature” is highly prognostic of poor survival in BC patients, demonstrating that the status of the tumor microenvironment represents an important variable for BC progression (26) and strongly suggesting the potential relevance of the wound response induced by surgery.
Studies performed in mice have shown the presence of growth stimulating factors in mouse serum after removal of the primary tumor (22) and recently the stimulatory effects of post-surgical drainage fluids harvested for 24 hours after surgery from BC patients (hereafter referred to as wound fluids, WF) have also been tested (27,28). Tagliabue et al. reported that WF, as well as postsurgical serum samples, induce proliferation of HER2-positive breast carcinoma cells, signifying that at the site of surgery growth factors able to induce BC cells proliferation are secreted (28). It has also been demonstrated that WF collected from EBC patients undergone breast surgery, stimulated cancer cell growth, migration and invasion (27), supporting the role of surgery as perturbing factor for recurrence formation, also in human patients.
Based on these observations, we pursued the identifications of the pathways specifically activated in BC residual cells by the wound response after surgery. Our very recent work highlighted the relevance in this context of two signaling pathways, the p70S6K and the STAT3 pathway.
p70S6K signaling and breast cancer (BC)
The PI3K/mTOR/p70S6K signaling axis is known to regulate many processes in the cells, many of which are critical for tumorigenesis, such as cell growth, proliferation, survival and metabolism (29). Briefly, the activation of PI3K (phosphatidylinositol 3-kinase) and the production of the lipid second messenger PIP3 (phosphatidylinositol 3,4,5-trisphosphate) from PIP2 trigger the recruitment and activation of Akt (protein kinase B) by the phosphatidylinositol-dependent kinases, PDK1 and PDK2 (30). One of the major downstream effectors of Akt is mTOR. Akt phosphorylates and inactivates the tuberous sclerosis complex (TSC) tumor suppressor, leading to the activation of the mammalian target of rapamycin complex 1 (mTORC1, hereafter mTOR) signaling pathway that results in the activation of p70S6K (31).
The ribosomal protein S6 kinase family comprises two homologous proteins, S6K1 and S6K2, each of which are found as two alternatively spliced isoforms (p70S6K and p85S6K, in the case of S6K1). Both S6K1 and S6K2 are downstream of mTOR pathway and exert same redundant functions. The p70 kDa isoform of S6K1 (p70S6K1) is the most studied S6 kinase, it is ubiquitously expressed and localizes predominantly to the cytoplasm (32). S6K1 plays important roles in cell growth, proliferation, and differentiation, mainly by regulating protein synthesis, cell cycle progression and metabolism (33-35).
It is extensively reported that p70S6K pathway is inappropriately activated in many cancer types, by receptor tyrosine kinases, as well as by the genetic mutation and overexpression of other key pathway components such as PI3K, Akt or loss of expression/function of negative regulators, such as the tumor suppressors PTEN and TSC1/2. The aberrant activation of this pathway plays a major role in BC and many evidences suggest that it is linked to promotion of BC cell growth and survival, resistance to chemotherapy, resistance to endocrine therapy and it is associated with poor prognosis, advanced stage and histological grade (36-38).
Recent studies have addressed the specific role of S6K1 in tumor proliferation, invasiveness, motility and angiogenesis (39,40). In particular, many data suggest the involvement of p70S6K1 also in BC onset and/or progression. p70S6K1 is encoded by the RPS6KB1 gene that is amplified in ~9% of primary breast tumors, leading to the overexpression of the protein (41-44). Moreover, RPS6KB1 amplification and overexpression are associated with poor prognosis in an unselected series of BC patients (43). Interestingly, activation of p70S6K1 (monitored by evaluation of phosphorylation status of Thr389 residue) has been found elevated by 10- to 35-folds in BC cells compared to normal primary mammary epithelial cells (45). Moreover, more than 70% of invasive breast carcinomas, have been demonstrated to possess high levels of phosphorylated p70S6K1 and, in sharp contrast, phosphorylation of the same protein was nearly undetectable in normal mammary tissues under the same assay (45). Furthermore, overexpression of p70S6K1 protein is linked to increased risk of locoregional recurrence in node-negative EBC patients, thus suggesting a role for this kinase also as prognostic marker (46).
Role of p70S6K signaling pathway in breast cancer (BC) recurrence
Using WF drained from BC patients after surgery as a model to study the impact of the wound response in recurrence formation, Segatto et al. recently demonstrated that BC cells responded to WF stimulation hyper-activating p70S6K pathway and this activation positively contributes to proliferation and invasion programs of BC epithelial cells, in vitro (47,48). Impairment of p70S6K1 signaling slightly decreased primary breast tumor growth in nude mice and an intact p70S6K1 signaling was extremely critical for tumor initiation, as demonstrated by tumor take-rate analyses. More interestingly, impairment of p70S6K1 signaling significantly increased the tumor latency (9 versus 26 days) but, once tumors appeared, their growth rate was very similar. This observation pointed out that, in the process of tumor initiation, p70S6K1 signaling played a major role in survival rather than in proliferation of BC cells. This hypothesis was also supported by the results from pharmacological inhibition of p70S6K1 activity in vivo demonstrating that a specific p70S6K1 inhibitor, PF-4708671, impairs cancer cell survival in hostile condition while having minor effects, if any on the growth of established tumors (47,48).
These data strongly support the observation that p70S6K1 plays a fundamental role in the formation of LR. Using a preclinical model that mimics the clinical setting Belletti and coworkers showed that interfering with p70S6K activity had a significant impact on BC cell behavior and almost completely prevented formation of local recurrence. Importantly, a three-day schedule of peri-operative treatment with the specific PF-4708671 was sufficient to significantly prevent the appearance of relapses, demonstrating that this critical event takes place in very narrow window of the disease (47). From a molecular point of view, the crosstalk between p70S6K signaling and the Hedgehog-Gli1 pathway was found to be crucial to activate the survival response needed by BC cells to escape apoptosis in critical contexts, as recently suggested also by others in other cancer types (48-50).
Importantly, activation of p70S6K1 after surgery takes place also in BC patients. In fact, in paired BC specimens from patients who underwent lumpectomy first and surgical widening to clear margins 1-2 weeks later, nearly 50% of patients displayed an increase of p70S6K1 activity in the second specimen respect to the first and only 8% showed a reverse trend, thus strongly supporting the hypothesis that p70S6K1 activity is increased by post-surgery stimuli (47).
Relevance of p70S6K1 in the survival of BC cells, more than in proliferation per se, is also supported by the work of Akar et al. demonstrating in a BC xenograft model that survival and engraftment of lung metastasis relies on p70S6K1 activity (51).
Inhibition of mTOR by temsirolimus should, in principle, elicit the same response of p70S6K1 inhibition in BC recurrence formation but this seemed not to be the case. Administration of temsirolimus did not reduce BC cell survival in vitro nor of local recurrence in vivo, probably because blocking the p70S6K negative feedback loop, led to paradoxical hyperactivation of AKT and up-regulation of Bcl2, which, in turn, fostered cell survival. This finding supported the notion that, under prolonged Tems treatment, isolated cells activated a survival response that is avoided when only p70S6K1 is specifically inhibited (47,48,52). These in vitro and preclinical data indicate that caution should be used in the administration of mTOR to BC patients since their use could lead to paradox activation of canonical survival pathways in residual BC cells.
Overall several works point to the activation of p70S6K1 as a key determinant of BC cell survival in the post-surgery microenvironment and suggest that impairing its activity could positively impact on prognosis of BC patients. Yet, specific and clinically tested p70S6K1 inhibitors will be necessary to transfer this knowledge to the human pathology.
STAT3 signaling in breast cancer (BC)
It has long been known that inflammatory conditions can initiate or promote oncogenic transformation and cancer-associated inflammation is marked by the abundant presence of specific inflammatory cells and inflammatory mediators, such as cytokines and chemokines (52). Recent evidences suggest a crucial role for signal transducer and activator of transcription (STAT) family proteins, especially STAT3, in selectively inducing and maintaining a pro-carcinogenic inflammatory microenvironment, during both initiation of malignant transformation and cancer progression (53,54). STAT3 belongs to the STAT family of proteins, which are both signal transducers and transcription factors. At least seven members of this family have been identified, encoded by distinct genes (55).
STAT3 plays important roles in fundamental processes, including proliferation, development, differentiation, inflammation and apoptosis (54,56-58). STAT3 is activated either by growth factor receptor, such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), or by non receptor tyrosine kinases, such as JAK or Src (59,60). Upon the binding of growth factors or cytokines to their cognate receptors on the cell surface, STAT3 is recruited to the cytoplasmic tail of the receptors and becomes phosphorylated on its Tyr 705. Tyrosine-phosphorylated STAT3 then dimerizes through reciprocal pTyr-SH2 domain interactions, translocates into the nucleus and binds to specific STAT-response elements in the promoters of target genes, thereby inducing the transcription of those genes essential for its physiological functions (61-64).
STAT3 regulates the transcription of several genes, involved in apoptosis, cell cycle and epithelial-mesenchymal transition (53,54,56-58). Under normal biological conditions, STAT3 activation is rapid and transient. However, it is to note that many of the downstream target genes of STAT3 encode for cytokines and growth factors, the receptors of which signal through the same STAT3, thereby providing a positive feed forward loop of autocrine and paracrine STAT3 activation (61,64-66). STAT3 has been found to be hyper-phosphorylated and constitutively activated in a large number of solid tumors and cancer cell lines, which often become addicted to its activity for continuous survival and growth (67,68).
The role of STAT3 in promoting and sustaining transformation is well documented. Conditional knockout of the STAT3 gene or inhibition of STAT3 function block v-Src induced transformation (69,70), indicating a pivotal role for STAT3 in malignant transformation. Moreover a constitutively dimerized STAT3 protein is sufficient to induce malignant transformation and tumor formation in mice (71). The exact mechanisms by which constitutively active STAT3 mediates malignant transformation and human tumor formation are still incompletely understood and continue to be investigated.
Constitutive activity of STAT3 has been observed in 35% to 60% of human breast tumors and in many BC cell lines, in which is required for continuous proliferation and resistance to apoptosis. Since no STAT3 mutations have been identified, constitutive activation of STAT3 in breast tumors is frequently associated with the aberrant expression and/or the activity of the EGF receptor family kinases, Src or JAK (72-76). The significance of STAT3 overexpression and/or activation in BC is however not completely clear and still debated (77,78).
Recently, high interest was raised by the possibility that STAT3 played a key role in regulating the self-renewal ability of BC stem cells (79,80). Cancer stem cells or tumor initiating cells (TICs) are rare cells and many studies support their involvement in tumor recurrence, formation of metastases, as well as chemoresistance (81,82). It was demonstrated that activated JAK2/STAT3 signaling is essential for the survival of CD44+/CD24-/low BC cells (83) and it was shown to play an important role during mammosphere formation, an in vitro assay used to isolate and propagate BC stem-like cells (84). STAT3 was also identified by an RNAi screen, as a critical player for mammosphere formation and self-renewal of breast TIC (85,86). Despite the fact that a sizable body of evidences highlight that STAT3 is inappropriately activated in a vast percentage of breast tumors, its concrete role in BC initiation and/or progression is still very controversial (87).
Since the discovery of the association between constitutive STAT3 activation and malignant transformation, a large number of studies have been undertaken for validating STAT3 as a cancer drug target (88-90), and substantial efforts were employed into the discovery of novel STAT3 inhibitors. A large number of STAT3 inhibitors have been developed, displaying different mechanism of actions. Of these inhibitors, a few of them has been validated and show good activity in terms of the inhibition of STAT3 biological functions and the associated antitumor cell effects, as well as the inhibition of tumor growth in the mouse models of human tumors (90). Up to now, these inhibitors are mostly at the experimental stage and only a few have been tested in clinical trials, with limited success (91-93).
Role of STAT3 signaling pathway in breast cancer (BC) recurrence
WFs are extremely rich in cytokines and growth factors and represent a surrogate source of the inflammatory stimuli present in the post-surgical setting in breast microenvironment. Strong and specific activation of STAT3 is induced in BC cell lines following WF stimulation. Moreover, WF-induced STAT3 activation was far more pronounced respect to activation induced by other mitogenic stimuli, indicating a specific role of STAT3 signaling pathway in this context (94).
In accord with the role proposed for STAT3 in the growth maintenance and self-renewal ability of TICs, Segatto et al. observed that WF is able to efficiently stimulate the cancer initiating phenotypes and self-renewal potential of BC cells (94). Genetic and/or pharmacological inhibition of STAT3 completely prevented self-renewal of BC cells stimulated with WF, suggesting that the inflammatory stimuli present in the post-surgical setting in breast microenvironment mediated TIC proliferation at least in part, via STAT3 activation (94).
In agreement with these observations, STAT3 activity not only positively impacted on the initiation of breast tumorigenesis in vivo, but, more importantly, was detrimental in the process of recurrence formation. In a mouse model of LR, the inhibition of STAT3 activity decrease the appearance of recurrences, suggesting that STAT3 activation plays a pivotal role in the regulation of the processes that lead to the re-growth of the tumor (94).
Wound response after intraoperative radiotherapy (IORT)
TARGIT-A trial was launched in 2000 to test whether IORT might be considered an alternative to external RT (20,95). IORT delivers a high dose of radiation as one single fraction at the time of surgery, allowing the precise application of radiation to the target area around the surgical bed. The clinical outcome of TARGIT application in EBC patients was recently reported, showing that the intraoperative treatment is more effective than previously hypothesized (95). From a molecular point of view, Belletti et al. evaluated whether treatment with intra-operative RT may reduce local recurrence by contextually killing residual tumor cells and affecting the peri-tumor microenvironment (27). Both in vitro results (27) and clinical observations (95) suggest that clinical success of TARGIT may be due, at least in part, to the alteration of the microenvironment through the modulation of the wound healing response induced by intra-operative RT. In fact, WF derived from TARGIT-treated patients were defective in activating both p70S6K and the STAT3 signaling pathways if compared with WF from patients treated only with surgery, while other pathways, such as AKT or ERK, were essentially unaffected (27).
These differences in pathway activation had functional consequences, since the stimulation of BC cell motility, invasion and growth observed with WF harvested from patients who have undergone wide local tumor excision was significantly abrogated when the WF were harvested from TARGIT treated patients (27).
A proteomic analysis on WF evaluating the levels of 174 cytokines, demonstrated that TARGIT treatment modified the levels of several factors involved in the control of cell growth and motility, such as IL-6, IL-8, HGF, UPA, Leptin and Rantes (27). Moreover, in accord with previous observation on the effects of RT on cytokines expression modification in humans and animals models (96-98), a specific increase in IL-5 and IL-4 following TARGIT was observed. This cytokine imbalance could eventually dictate a different immune response in local microenvironment, eventually amplifying the anti-tumoral effects of IORT. In line with this hypothesis, the modification of immune response by RT has been recently proposed (99,100). Moreover, it has been recently reported that application of IORT to BC patients induces a rapid and reproducible modification of microRNA expression that could eventually modify the crosstalk between residual tumor cells and the post-surgery microenvironment thus participating to the antitumor response of IORT (101).
Overall, it is thus possible that intra-operative radiotherapic treatments, such as TARGIT, in addition to the conventionally known tumoricidal effect exert their therapeutic effects by altering the tumor microenvironment, eventually leading to reduce recurrence formation (Figure 1).
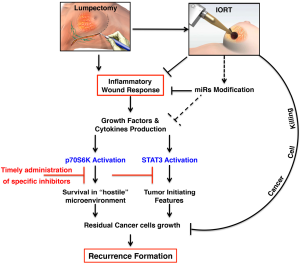
A large body of literature exists on the possible unwanted and harmful effects of RT. These include onset of cardiac toxicity (102) and appearance of second tumors (103) and, also, the paradoxical stimulation of tumor cell growth and spreading, attributed to modifications of the local microenvironment. It is known, for instance, that RT induces a hypoxic condition, which, in turn, may stimulate tumor neoangiogenesis (104,105) and promote the production of pro-metastatic growth factors, such as TGFβ (106). Whether these harmful effects are related to the dose, the modality, the timing and the type of RT delivery is largely unknown. At this regard, it is possible to speculate that the effects of RT could greatly differ if radiation is applied to a wounded tissue respect to the application from the outside, to an already repaired breast, as it is in the case of EBRT during BC therapy. The generation of appropriate models of BC recurrence coupled with model of precise irradiation on wounded and intact breasts will be necessary to verify whether these hypotheses are true.
Discussion
The possible harmful effects of surgical wounding have been speculated for a long time and have been demonstrated in mice (13,18). Also in humans, it was demonstrated that the growth kinetics of BC micro metastasis was modified by surgery, representing a perturbing factor in the process of relapse or metastasis development (19,26). Moreover, experimental and clinical observations suggest that the extent of surgery may represent a variable able to enhance tumor burden (14,17-19,107).
So, surgery and the consequent inflammatory response caused by wounding could represent factors that favor the proliferation of “residual” tumor cells. The communication between microenvironment and tumor cells plays an important role in this context. At initial stages of tumor development, the microenvironment surrounding the tumor should provide tumor-suppressive signals; however, once tissue homeostasis is lost, the altered microenvironment can itself become a potent tumor promoter, as widely demonstrated in literature (108). The process of wound healing can induce changes in the microenvironment, such that a shift the balance between tumor-suppressive and tumor-promoting signals occurs. The combination of post surgery inflammation with wound healing-induced growth factor production can breach the barrier, resulting in promotion of the growth of residual cells into a tumor.
Accordingly, wound axillary fluids harvested from BC patients have been proved to stimulate Her2-positive mammary carcinoma cell growth and this effect is only partially abrogated by impairing Her2 signal transduction (28). Our work, formally demonstrating that WF harvested from BC patients who have undergone wide local tumor excision strongly stimulate BC cell growth, motility and invasion, also shows for the first time that one single application of IORT with TARGIT is sufficient to significantly abrogate the stimulatory effects of surgical WF on cancer cells in vitro. These findings strongly support the idea that TARGIT may confer more benefits than those expected merely from the tumoricidal effect of RT (27). In support to this hypothesis the recent results of randomized trial TARGIT-A confirmed non-inferiority clinical outcomes respect EBRT, only when TARGIT was delivered concurrently with lumpectomy (prepathology stratum) but not when it was delivered at a later time, through a second surgical procedure (postpathology stratum) (95). Several factors might have played a part in achieving the low recurrence rates that was identified in the stratum randomized to receive TARGIT concurrently to lumpectomy. The immediate delivery of radiation to the wounded tissue appears to be essential to achieve the beneficial effects on the tumor microenvironment, suggesting that the timing of treatment is an important variable that can determine different clinical response (95), in line with the findings reporting that TARGIT delivery significantly modifies the protein expression profile of the WF (27), the activation of signaling pathways in BC cells and also microRNA transcription and secretion (101).
The act of surgery leads to a profound modification of the local microenvironment. In that context, reactive microenvironment is able to sustain the survival and, eventually, the re-growth of residual cancer cells through the secretion of inflammatory cytokines and growth factors. Altogether, many results allow to suggest that the signaling pathways that strongly influence the response of residual BC cells are mainly two. The activation of p70S6K1 prevalently fosters the survival of these cells, while the activation of STAT3 supports the self-renewal ability of breast TICs. We can hypothesize that these pathways cooperate in the post-surgical setting to allow the survival and re-growth of residual cells, eventually leading to the formation of BC recurrence.
Taken together, the findings presented in this review of the literature provide a biological rationale for the use of molecularly targeted agents to compensate the harmful consequences of surgery. It is well recognized that improved clinical efficacy of RT represents a substantial progress in clinical practice and patient outcomes (109). Thus, the use of peri-operative targeted treatments in combination with IORT could improve the clinical response in EBC patients through the “sterilization” of microenvironment and the subsequent inhibition of the pathway mainly involved in recurrence formation, such as p70S6K and STAT3. We propose that this combination treatment, coupled with the correct timing of administration, should be soon tested to improve patient response, particularly in those BC subtypes in which response to standard therapies is currently low.
Acknowledgments
We wish to thank all members of the SCICC lab in the Division of Experimental Oncology 2, CRO Aviano, for scientific and technical contributions.
Funding: This work is supported by CRO Intramural Research grant to S Massarut and G Baldassarre; AIRC IG 15902 to B Belletti. I Segatto is a recipient of a FIRC research fellowship.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frank A. Giordano, Pedro Carlos Lara and Frederik Wenz) for the series “Intraoperative Radiotherapy II” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.04.02). The series “Intraoperative Radiotherapy II” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer 2005;6:391-401. [PubMed]
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784-92. [PubMed]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer 2007;7:659-72. [PubMed]
- Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest 2011;121:3797-803. [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [PubMed]
- Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5:5-23. [PubMed]
- Perou CM, Børresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol 2011;3:a003293 [PubMed]
- Benson JR, Jatoi I, Keisch M, et al. Early breast cancer. Lancet 2009;373:1463-79. [PubMed]
- Komoike Y, Akiyama F, Iino Y, et al. Analysis of ipsilateral breast tumor recurrences after breast-conserving treatment based on the classification of true recurrences and new primary tumors. Breast Cancer 2005;12:104-11. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [PubMed]
- Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst 2008;100:1179-83. [PubMed]
- Fisher B, Anderson S, Fisher ER, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet 1991;338:327-31. [PubMed]
- Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 1995;87:19-27. [PubMed]
- Whelan T, Clark R, Roberts R, et al. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys 1994;30:11-6. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [PubMed]
- Baker DG, Masterson TM, Pace R, et al. The influence of the surgical wound on local tumor recurrence. Surgery 1989;106:525-32. [PubMed]
- Demicheli R, Valagussa P, Bonadonna G. Does surgery modify growth kinetics of breast cancer micrometastases? Br J Cancer 2001;85:490-2. [PubMed]
- Demicheli R, Retsky MW, Hrushesky WJ, et al. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol 2007;4:699-710. [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [PubMed]
- Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 1979;39:3861-5. [PubMed]
- Fisher B, Gunduz N, Coyle J, et al. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res 1989;49:1996-2001. [PubMed]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650-9. [PubMed]
- Iozzo RV. Tumor stroma as a regulator of neoplastic behavior. Agonistic and antagonistic elements embedded in the same connective tissue. Lab Invest 1995;73:157-60. [PubMed]
- Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996;76:69-125. [PubMed]
- Troester MA, Lee MH, Carter M, et al. Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res 2009;15:7020-8. [PubMed]
- Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325-32. [PubMed]
- Tagliabue E, Agresti R, Carcangiu ML, et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet 2003;362:527-33. [PubMed]
- Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov 2010;5:29-57. [PubMed]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010;11:9-22. [PubMed]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004;23:3151-71. [PubMed]
- Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol 2011;43:47-59. [PubMed]
- Düvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 2010;39:171-83. [PubMed]
- Shin S, Wolgamott L, Yu Y, et al. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc Natl Acad Sci U S A 2011;108:E1204-13. [PubMed]
- Kawasome H, Papst P, Webb S, et al. Targeted disruption of p70(s6k) defines its role in protein synthesis and rapamycin sensitivity. Proc Natl Acad Sci U S A 1998;95:5033-8. [PubMed]
- Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci 2005;10:975-87. [PubMed]
- Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005;4:988-1004. [PubMed]
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005;24:7455-64. [PubMed]
- Skinner HD, Zheng JZ, Fang J, et al. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J Biol Chem 2004;279:45643-51. [PubMed]
- Zhou HY, Wong AS. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology 2006;147:2557-66. [PubMed]
- Couch FJ, Wang XY, Wu GJ, et al. Localization of PS6K to chromosomal region 17q23 and determination of its amplification in breast cancer. Cancer Res 1999;59:1408-11. [PubMed]
- Monni O, Barlund M, Mousses S, et al. Comprehensive copy number and gene expression profiling of the 17q23 amplicon in human breast cancer. Proc Natl Acad Sci U S A 2001;98:5711-6. [PubMed]
- Bärlund M, Forozan F, Kononen J, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst 2000;92:1252-9. [PubMed]
- Sinclair CS, Rowley M, Naderi A, et al. The 17q23 amplicon and breast cancer. Breast Cancer Res Treat 2003;78:313-22. [PubMed]
- Lin HJ, Hsieh FC, Song H, et al. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br J Cancer 2005;93:1372-81. [PubMed]
- van der Hage JA, van den Broek LJ, Legrand C, et al. Overexpression of P70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer 2004;90:1543-50. [PubMed]
- Segatto I, Berton S, Sonego M, et al. Inhibition of breast cancer local relapse by targeting p70S6 kinase activity. J Mol Cell Biol 2013;5:428-31. [PubMed]
- Segatto I, Berton S, Sonego M, et al. p70S6 kinase mediates breast cancer cell survival in response to surgical wound fluid stimulation. Mol Oncol 2014;8:766-80. [PubMed]
- Wang Y, Ding Q, Yen CJ, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell 2012;21:374-87. [PubMed]
- Filbin MG, Dabral SK, Pazyra-Murphy MF, et al. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nat Med 2013;19:1518-23. [PubMed]
- Akar U, Ozpolat B, Mehta K, et al. Targeting p70S6K prevented lung metastasis in a breast cancer xenograft model. Mol Cancer Ther 2010;9:1180-7. [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [PubMed]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798-809. [PubMed]
- Calò V, Migliavacca M, Bazan V, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 2003;197:157-68. [PubMed]
- Bromberg J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res 2000;2:86-90. [PubMed]
- Schindler C, Darnell JE. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 1995;64:621-51. [PubMed]
- Darnell JE. STATs and gene regulation. Science 1997;277:1630-5. [PubMed]
- Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci 2000;25:496-502. [PubMed]
- Grandis JR, Drenning SD, Chakraborty A, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest 1998;102:1385-92. [PubMed]
- Trevino JG, Gray MJ, Nawrocki ST, et al. Src activation of Stat3 is an independent requirement from NF-kappaB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis 2006;9:101-10. [PubMed]
- Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994;264:95-8. [PubMed]
- Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994;264:1415-21. [PubMed]
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 2007;282:20059-63. [PubMed]
- Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3:651-62. [PubMed]
- Heinrich PC, Behrmann I, Müller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 1998;334:297-314. [PubMed]
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007;7:41-51. [PubMed]
- Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 2008;18:254-67. [PubMed]
- Bowman T, Garcia R, Turkson J, et al. STATs in oncogenesis. Oncogene 2000;19:2474-88. [PubMed]
- Turkson J, Bowman T, Garcia R, et al. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 1998;18:2545-52. [PubMed]
- Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res 2005;65:5828-34. [PubMed]
- Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell 1999;98:295-303. [PubMed]
- Furth PA. STAT signaling in different breast cancer sub-types. Mol Cell Endocrinol 2014;382:612-5. [PubMed]
- Garcia R, Yu CL, Hudnall A, et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ 1997;8:1267-76. [PubMed]
- Garcia R, Bowman TL, Niu G, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 2001;20:2499-513. [PubMed]
- Diaz N, Minton S, Cox C, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res 2006;12:20-8. [PubMed]
- Berishaj M, Gao SP, Ahmed S, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res 2007;9:R32. [PubMed]
- Berclaz G, Altermatt HJ, Rohrbach V, et al. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol 2001;19:1155-60. [PubMed]
- Dolled-Filhart M, Camp RL, Kowalski DP, et al. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res 2003;9:594-600. [PubMed]
- Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol 2010;28:4006-12. [PubMed]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011;121:3804-9. [PubMed]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [PubMed]
- Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100:672-9. [PubMed]
- Marotta LL, Almendro V, Marusyk A, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24− stem cell-like breast cancer cells in human tumors. J Clin Invest 2011;121:2723-35. [PubMed]
- Hernandez-Vargas H, Ouzounova M, Le Calvez-Kelm F, et al. Methylome analysis reveals Jak-STAT pathway deregulation in putative breast cancer stem cells. Epigenetics 2011;6:428-39. [PubMed]
- Dave B, Landis MD, Tweardy DJ, et al. Selective small molecule Stat3 inhibitor reduces breast cancer tumor-initiating cells and improves recurrence free survival in a human-xenograft model. PloS One 2012;7:e30207 [PubMed]
- Wolf J, Dewi DL, Fredebohm J, et al. A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype. Breast Cancer Res 2013;15:R109. [PubMed]
- Resemann HK, Watson CJ, Lloyd-Lewis B. The Stat3 paradox: a killer and an oncogene. Mol Cell Endocrinol 2014;382:603-11. [PubMed]
- Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets 2004;8:409-22. [PubMed]
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer 2004;4:97-105. [PubMed]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs 2009;18:45-56. [PubMed]
- Bendell JC, Hong DS, Burris HA, et al. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol 2014;74:125-30. [PubMed]
- Sen M, Thomas SM, Kim S, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov 2012;2:694-705. [PubMed]
- Peyser ND, Grandis JR. Critical analysis of the potential for targeting STAT3 in human malignancy. Onco Targets Ther 2013;6:999-1010. [PubMed]
- Segatto I, Berton S, Sonego M, et al. Surgery-induced wound response promotes stem-like and tumor-initiating features of breast cancer cells, via STAT3 signaling. Oncotarget 2014;5:6267-79. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Yamazaki H, Inoue T, Tanaka E, et al. Pelvic irradiation-induced eosinophilia is correlated to prognosis of cervical cancer patients and transient elevation of serum interleukin 5 level. Radiat Med 2005;23:317-21. [PubMed]
- Jones BM, Kwok CC, Kung AW. Effect of radioactive iodine therapy on cytokine production in Graves’ disease: transient increases in interleukin-4 (IL-4), IL-6, IL-10, and tumor necrosis factor-alpha, with longer term increases in interferon-gamma production. J Clin Endocrinol Metab 1999;84:4106-10. [PubMed]
- Büttner C, Skupin A, Reimann T, et al. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol 1997;17:315-25. [PubMed]
- Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res 2008;10:215. [PubMed]
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718-26. [PubMed]
- Fabris L, Berton S, Massarut S, et al. Radiotherapy-induced miR expression influences the formation of local recurrence in breast cancer. Cancer Res 2012;72:P5-10-17.
- Schmitz KH, Prosnitz RG, Schwartz AL, et al. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer 2012;118:2270-6. [PubMed]
- Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys 2013;86:224-33. [PubMed]
- Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011;11:239-53. [PubMed]
- Kuonen F, Secondini C, Rüegg C. Molecular pathways: emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironment. Clin Cancer Res 2012;18:5196-202. [PubMed]
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer 2005;5:867-75. [PubMed]
- Tsuchiya Y, Sawada S, Yoshioka I, et al. Increased surgical stress promotes tumor metastasis. Surgery 2003;133:547-55. [PubMed]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011;17:320-9. [PubMed]
- Lin SH, George TJ, Ben-Josef E, et al. Opportunities and challenges in the era of molecularly targeted agents and radiation therapy. J Natl Cancer Inst 2013;105:686-93. [PubMed]


