
A newly improved POSSUM scoring system for prediction of morbidity in patients with pancreaticoduodenectomy
Introduction
Despite the advancement of surgical techniques and support from perioperative monitoring, perioperative mortality after PD has become a rare event with mortality rates below 5% (1,2). However, the post-PD morbidity rate remains high (38–44%) (2-4), and in recent decades, this situation has not improved. In addition, it is much higher than the incidence of postoperative complications in other gastrointestinal cancer surgery (5-7). In order to reduce the potential for complications following PD to develop, surgeons have proposed various surgical techniques; however, they cannot eliminate the possibility of complications. On the other hand, patients who suffered from one complication have a risk of subsequent complications, which indicates higher medical costs, a longer hospital stay, and severe impact on their health. These negative results of postoperative complications indicate the importance of calculating patient risk factors for better understanding of prevention strategies and early intervention.
Although the risk factors of complication following PD have been identified (8,9), few studies have established a scoring system. Moreover, the POSSUM scoring system is used to estimate general surgery and does not factor in issues specific to PD surgery. Moreover, most of their reports are based at a single institution, so it may not be possible to replicate these results at other institutions, and various biases should be taken into account when these results involve individual institutions or patients. To predict morbidity rate after PD and thus minimize it, we analyzed all variables and modified POSSUM score system to specific to PD surgery, which we call PD-POSSUM. Our findings provide a scientific basis for managing and preventing post-PD complications. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-828).
Methods
Ethics and informed consent
The research conformed to the Declaration of Helsinki (as revised in 2013) and it was authorized by the Human Ethics and Research Ethics Committees of the Fourth Hospital of Hebei Medical University (ID: 2016MEC078). The informed consent was approved by all that participates.
Recruitment of participates
A total of 201 patients underwent PD during January 2016 to December 2018 for periampullary diseases at the No.4 Hospital of Hebei Medical University in Shijiazhuang City, China. In addition, we defined all 201 cases as our modeling group.
Collection of perioperative parameters
In this study, a series of potentially predictive variables were associated with post-PD complications. Patient variables, included in the POSSUM scoring system (Table S1) are: cardiac signs, respiratory signs, electrocardiography, systolic blood pressure, pulse, hemoglobin, white cell count, urea, sodium, potassium, Glasgow coma scores were considered. In addition, several risk factors were included which are not factored into the POSSUM scoring system: platelet count, prothrombin time, body mass index (BMI), pre-existing respiratory disease, activated partial thromboplastin time, International normalized ratio of prothrombin time, alanine aminotransferase, albumin level, total bilirubin level, CA19-9, duration of surgery, diameter of bile duct, diameter of pancreatic duct, texture of pancreas level, and tumor diameter. We reviewed CT images to measure pancreatic duct size and bile duct size at portal vein level. Finally, BMI, the pre-existing comorbidity of respiratory diseases, active liver function protection, total bilirubin level, diameter of pancreatic duct, tumor diameter, and international normalized ratio of prothrombin time, which are not included in the POSSUM scoring system (Table S1 and Table S2), were assessed to predict patients’ postoperative outcome.
Outcomes measurement
The outcome measure for this study was the incidence of complications. Post-PD complications included stress ulcer, pancreatic fistula (PF), gastrointestinal bleeding, pleural effusion, intraabdominal infection and abscess, pneumonia, arrhythmia, hemorrhage, acute renal failure, even though mortality, delayed gastric emptying. The severity of complication was classified according to Clavien-Dindo classification grade. Clavien-Dindo classification grade II or more was regarded as significant (10).
Statistical analysis
The results are expressed as percentages and sample size (Table 1). For correlation analysis, the Spearman rho test was used for comparing pre- and intra-operative variables (Table 2). All test results were then entered into a multivariable linear regression model for identifying independently predictive factors of the complication (Table 3). Risk factors were entered into the same model. The complication values were then converted to natural log equivalents for statistical analysis. The variance expansion factor was calculated by a multiple linear regression model to quantify the severity of multicollinearity. Multiple logistic regression analyses (backward) were performed to calculate the odds ratio (OR) for each variable in the subject of the complication based on the criteria for complications (Table 4). At the end of this study, logistic regression analysis was performed to explore the connection between scorePD-POSSUM (see below) and morbidity rate (Table 5). A receiver operating characteristic (ROC) curve analysis was performed to determine the ability of scorePD-POSSUM to predict complications (Figure 1).
Table 1
| Risk factors | Complication | P | |
|---|---|---|---|
| Yes, n (%) | No, n (%) | ||
| Platelet count (×109/L) | |||
| ≤100 | 2 (1.0) | 1 (0.5) | |
| 100–300 | 67 (33.3) | 82 (40.8) | |
| >300 | 23 (11.4) | 26 (12.9) | |
| Prothrombin time (s) | |||
| ≤11 | 51 (25.4) | 61 (30.3) | |
| 11–13 | 31 (15.4) | 38 (18.9) | |
| 13–15 | 9 (4.5) | 8 (4.0) | |
| >15 | 1 (0.5) | 2 (1.0) | |
| BMI (kg/m2) | 0.000* | ||
| ≤18.5 | 5 (2.5) | 12 (6.0) | |
| 18.5–23.9 | 39 (19.4) | 68 (33.8) | |
| 24–27.9 | 42 (20.9) | 28 (13.9) | |
| >28 | 6 (3.0) | 1 (0.5) | |
| Pre-existing respiratory disease | 0.001* | ||
| Yes | 54 (26.9) | 45 (22.4) | |
| No | 38 (18.9) | 64 (31.8) | |
| Activated partial thromboplastin time (s) | |||
| ≤43.50 | 88 (44.0) | 105 (52.5) | |
| >43.50 | 4 (2.0) | 3 (1.5) | |
| International normalized ratio of prothrombin time (s) | 0.058 | ||
| ≤1.10 | 85 (42.3) | 95 (47.3) | |
| >1.10 | 7 (3.5) | 14 (7.0) | |
| Alanine aminotransferase (U/L) | 0.010* | ||
| ≤40 | 14 (7.0) | 18 (9.0) | |
| 40–200 | 49 (24.4) | 35 (17.4) | |
| 200–400 | 18 (9.0) | 42 (20.9) | |
| >400 | 11 (5.5) | 14 (7.0) | |
| Albumin level (g/L) | |||
| ≤20 | 1 (0.5) | 1 (0.5) | |
| 20–28 | 11 (5.5) | 9 (4.5) | |
| 28–40 | 65 (32.3) | 75 (37.3) | |
| >40 | 15 (7.5) | 24 (11.9) | |
| Total bilirubin level (μmol/L) | 0.018* | ||
| ≤17.1 | 10 (5.0) | 20 (10.0) | |
| 17.1–34.2 | 8 (4.0) | 8 (4.0) | |
| 34.2–171.0 | 34 (16.9) | 42 (20.9) | |
| 171.0–342.0 | 31 (15.4) | 33 (16.4) | |
| >342 | 9 (4.5) | 6 (3.0) | |
| CA19-9 (kU/L) | |||
| ≤40 | 23 (11.4) | 26 (12.9) | |
| >40 | 69 (34.3) | 83 (41.3) | |
| Duration of surgery (h) | |||
| ≤5 | 43 (21.4) | 63 (31.3) | |
| 5–6 | 40 (19.9) | 36 (17.9) | |
| 6–8 | 7 (3.5) | 10 (5.0) | |
| >8 | 2 (1.0) | 0 (0.0) | |
| Diameter of bile duct (cm) | |||
| ≤1 | 7 (3.5) | 13 (6.5) | |
| 1–3 | 84 (41.8) | 95 (47.3) | |
| >3 | 1 (0.5) | 1 (0.5) | |
| Diameter of pancreatic duct (mm) | 0.004* | ||
| <3 | 76 (37.8) | 53 (26.3) | |
| 3–5 | 32 (15.9) | 39 (19.4) | |
| >5 | 0 (0.0) | 1 (0.5) | |
| Texture of pancreas level | |||
| 1 | 45 (22.4) | 51 (25.4) | |
| 2 | 11 (5.5) | 7 (3.5) | |
| 3 | 21 (10.4) | 26 (12.9) | |
| 4 | 4 (2.0) | 5 (2.5) | |
| 5 | 11 (5.5) | 20 (10.0) | |
| Tumor diameter (cm) | 0.012* | ||
| ≤2 | 35 (17.4) | 58 (28.9) | |
| >2 | 57 (28.4) | 51 (25.4) | |
| Total | 92 (45.5) | 109 (54.2) | |
*, P<0.05.
Table 2
| Characteristics | Complication | ||
|---|---|---|---|
| ρ# | P | χ2 | |
| Platelet count | 0.002 | 0.977 | 0.594 |
| Prothrombin time | 0.011 | 0.877 | 0.561 |
| BMI | 0.270** | 0.000** | 15.789** |
| Pre-existing respiratory disease | 0.174* | 0.014* | 6.051** |
| Activated partial thromboplastin time | 0.043 | 0.549 | 0.363 |
| International normalized ratio of prothrombin time | −0.085 | 0.229 | 1.462 |
| Alanine aminotransferase | −0.126 | 0.074 | 11.437** |
| Albumin level | −0.087 | 0.217 | 1.565 |
| Total bilirubin level | 0.105 | 0.140 | 3.425 |
| CA19-9 | −0.013 | 0.851 | 0.036 |
| Duration of surgery | 0.101 | 0.152 | 5.112** |
| Diameter of bile duct | 0.071 | 0.319 | 1.046 |
| Diameter of pancreatic duct | 0.149* | 0.035* | 5.941** |
| Texture of pancreas level | −0.063 | 0.376 | 3.104 |
| Tumor diameter | 0.152** | 0.032** | 4.617** |
#, Spearman rank correlation coefficient between complication and characteristics; ρ: Spearman correlation coefficient; χ2: Pearson Chi-square; *,**, significant variables. BMI, body mass index.
Table 3
| Characteristics | Complications | ||
|---|---|---|---|
| β& | P | VIF | |
| First step | |||
| Platelet count | 0.007 | 0.922 | 1.143 |
| Prothrombin time | 0.013 | 0.878 | 1.615 |
| BMI | 0.239 | 0.001 | 1.120 |
| Pre-existing respiratory disease | 0.194 | 0.004 | 1.062 |
| Activated partial thromboplastin time | 0.033 | 0.619 | 1.076 |
| International normalized ratio of prothrombin time | −0.141 | 0.072 | 1.451 |
| Alanine aminotransferase | −0.200 | 0.013 | 1.506 |
| Albumin level | −0.007 | 0.926 | 1.278 |
| Total bilirubin level | 0.143 | 0.107 | 1.849 |
| CA19-9 | 0.003 | 0.972 | 1.246 |
| Duration of surgery | 0.086 | 0.210 | 1.105 |
| Diameter of bile duct | 0.054 | 0.548 | 1.924 |
| Diameter of pancreatic duct | 0.201 | 0.005 | 1.200 |
| Texture of pancreas level | −0.097 | 0.148 | 1.062 |
| Tumor diameter | 0.173 | 0.017 | 1.237 |
| Last step | |||
| BMI | 0.253 | 0.000 | 1.059 |
| Pre-existing respiratory disease | 0.207 | 0.002 | 1.031 |
| International normalized ratio of prothrombin time | −0.114 | 0.083 | 1.029 |
| Alanine aminotransferase | −0.188 | 0.013 | 1.356 |
| Total bilirubin level | 0.174 | 0.019 | 1.321 |
| Diameter of pancreatic duct | 0.193 | 0.004 | 1.075 |
| Tumor diameter | 0.171 | 0.011 | 1.094 |
&, multiple linear regression analysis; β: Parameter estimate. BMI, body mass index.
Table 4
| Characteristics | Complications | ||
|---|---|---|---|
| OR | 95% CI | P | |
| First step | |||
| Platelet count | 1.038 | 0.486–2.217 | 0.924 |
| Prothrombin time | 1.106 | 0.614–1.990 | 0.738 |
| BMI | 2.578 | 1.491–4.457 | 0.001* |
| Pre-existing respiratory disease | 2.858 | 1.444–5.657 | 0.003* |
| Activated partial thromboplastin time | 1.473 | 0.273–7.962 | 0.653 |
| International normalized ratio of prothrombin time | 0.252 | 0.060–1.053 | 0.059 |
| Alanine aminotransferase | 0.556 | 0.356–0.869 | 0.010* |
| Albumin level | 1.021 | 0.545–1.910 | 0.949 |
| Total bilirubin level | 1.366 | 0.918–2.032 | 0.124 |
| CA19-9 | 1.058 | 0.458–2.448 | 0.895 |
| Duration of surgery | 1.339 | 0.820–2.186 | 0.243 |
| Diameter of bile duct | 1.545 | 0.369–6.477 | 0.552 |
| Diameter of pancreatic duct | 1.847 | 1.202–2.839 | 0.005* |
| Texture of pancreas level | 0.867 | 0.693–1.084 | 0.211 |
| Tumor diameter | 2.387 | 1.149–4.957 | 0.020* |
| Last step | |||
| BMI | 2.700 | 1.594–4.572 | 0.000* |
| Pre-existing respiratory disease | 3.000 | 1.542–5.837 | 0.001* |
| International normalized ratio of prothrombin time | 0.321 | 0.099–1.038 | 0.058 |
| Alanine aminotransferase | 0.573 | 0.375–0.874 | 0.010* |
| Total bilirubin level | 1.477 | 1.068–2.043 | 0.018* |
| Diameter of pancreatic duct | 1.837 | 1.221–2.763 | 0.004* |
| Tumor diameter | 2.369 | 1.207–4.650 | 0.012* |
*P<0.05. 95% CI, 95% confidence interval; OR, odds ratio; BMI, body mass index.
Table 5
| Score type | Complication | Hosmer and Lemeshow test | ||||
|---|---|---|---|---|---|---|
| β | P | Constant quantity | Chi-square | Sig. | ||
| ScorePS | 0.140 | 0.000 | – | |||
| ScoreOS | −0.053 | 0.739 | ||||
| ScorePD-Possum | 0.998 | 0.000 | −0.022 | 6.766 | 0.562 | |
| SocrePossum | 0.213 | 0.377 | −0.571 | 5.795 | 0.564 | |
*, significant variables. β, parameter estimate. PS, physiological score; OS, operative score.
All statistical analyses were performed using SPSS software (version 21.0; IBM Corp, Beijing, China). A P value <0.05 was considered statistically significant.
Prediction model construction
Statistically significant factors for complication were identified by multivariate logistic regression model as well as in scorePD-POSSUM. Each index in the multiple logistic regression models was taken as the score of the corresponding index. Next, we added these scores to ScoreOS. The final equation of scorePD-POSSUM was obtained by adding the ScorePS and ScoreOS (Table S2):
Logistic regression analysis showed that scorePD-POSSUM was significantly associated with morbidity rate (R) (P=0.000, Table 5).
We then derived scorePOSSUM (Table S1) for performing comparisons with scorePD-POSSUM scoring systems that have been shown to be associated with postoperative morbidity rate:
Results
The pre- and intra-operative characteristics (P<0.05) of the 201 patients in the modeling group are listed in Table 1. Of the 201 patients enrolled in this study, morbidity rate in the modeling group was 45.5% (Table 1), resulting from: stress ulcer (15 cases), pancreatic fistula (PF, 34 cases), gastrointestinal bleeding (20 cases), pleural effusion (3 cases), intraabdominal infection and abscess (35 cases), pneumonia (8 cases), arrhythmia (3 cases), acute renal failure (1 case) and death (2 cases). Table 1 also summarizes the association between complication and pre- and intra-operative characteristics of the patients as well as the complication in accordance with pre- and intra-operative characteristics. Complication was significantly and positively associated with BMI (P=0.000), pre-existing comorbidity of respiratory diseases (P=0.001), alanine aminotransferase (P=0.010), total bilirubin level (P=0.018), diameter of pancreatic duct (P=0.004), and tumor diameter (P=0.012).
To confirm that BMI, the pre-existing comorbidity of respiratory diseases, alanine aminotransferase, and total bilirubin level, diameter of pancreatic duct and tumor diameter have an impact on post-PD complications, we further analyzed the correlation of the morbidity after PD with associated risk factors by calculating Spearman correlation coefficients (Table 2). In the multivariate linear regression (backward), natural logarithm complications are still associated with BMI (P=0.000) in the case where all other variables being held at fixed values, the pre-existing comorbidity of respiratory diseases (P=0.002), alanine aminotransferase (P=0.013), total bilirubin level (P=0.019), diameter of pancreatic duct (P=0.004), and tumor diameter (P=0.011), and the program retained the international normalized ratio of prothrombin time (P=0.083) during the backward process (Table 3).
We conducted multivariate logistic regression analysis to identify the risk factor among these variables. Table 4 summarizes the subjects’ multivariate ORs and 95% confidence intervals (95% CIs). The OR for complication was 2.700 (95% CI, 1.594–4.572) in risk factor with BMI. Relative to variables that have the pre-existing comorbidity of respiratory diseases, the adjusted OR of complication was 3.000 (95% CI, 1.542–5.837). And relative to characteristics of international normalized ratio of prothrombin time, the OR for complication was 0.321 (95% CI, 0.099–1.038). Variables with higher total bilirubin level had significantly higher morbidity rate than those with lower one (OR, 1.477; 95% CI, 1.068–2.043). In terms of diameter of pancreatic duct and tumor diameter, the ORs for complication were 1.837 (95% CI, 1.221–2.763) and 2.369 (95% CI, 1.207–4.650) respectively (P<0.05). Finally, univariate logistic regression analysis was performed to explore the logistic correlation between scorePD-POSSSUM and the complication (Table 5).
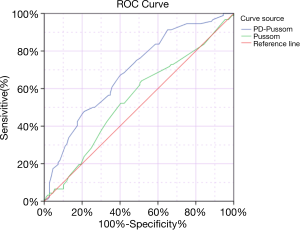
We constructed ROC curves to determine an accurate threshold of scorePD-POSSUM for predicting complications (Table 6). ScorePD-POSSUM was primarily associated with a higher risk of complications [area under the curve (AUC) for complication, 0.689; 95% CI, 0.616–0.762; P=0.000]. However, scorePOSSUM (AUC for complication, 0.545) was not sensitive enough to predict complication when comparing with scorePD-POSSUM.
Table 6
| Score type | Complication | ||
|---|---|---|---|
| AUC | P | 95% CI | |
| ScorePD-possum | 0.689 | 0.000* | 0.616–0.762 |
| Scorepossum | 0.545 | 0.271 | 0.465–0.625 |
*, significant variables. AUC, area under curve.
Discussion
The present study focused on the patients with post-PD complications. For the 201 patients undergoing PD recorded in the study, morbidity was still high at 45.5%. Regarding its predictions, thanks to previous studies, a number of factors have been identified to predict the occurrence of complications, such as indication disease for the surgical treatment, BMI, active liver function protection, and total bilirubin level, diameter of pancreatic duct, tumor diameter and international normalized ratio of prothrombin time. However, few studies have focused on establishing formulas to estimate and predict whether complications will, in fact, occur. We successfully identified seven statistically significant risk factors associated with post-PD complications, including: BMI, the pre-existing comorbidity of respiratory diseases, active liver function protection, total bilirubin level, diameter of pancreatic duct, tumor diameter, and international normalized ratio of prothrombin time.
While these risk factors were assessed using the POSSUM scoring system for the first time, some studies have shown a significant link between these factors and complications. For example, El Nakeeb et al. (11) analyzed 471 cases of pancreaticoduodenectomy and found that BMI >25 was a risk factor for postoperative pancreatic fistula (POPF). Gaujoux et al. (12) analyzed 100 successive cases of pancreaticoduodenectomy and similarly found that BMI >25 was a risk factor for pancreatic fistula after pancreaticoduodenectomy. And POPF is one of the most serious complications following PD. The higher incidence of post-PD complications in patients with increased BMI may be associated with the following factors: increased difficulty in exposing the pancreas during surgery cause by a higher volume of abdominal fat and peripancreatic fat, a higher risk of damage to the pancreatic capsule during separation attributed to a soft and brittle pancreas, and a higher risk of pancreatic leakage due to damage to the pancreatic tissue and fine pancreatic ducts cause by suturing and knotting during pancreaticojejunal anastomosis. With regard to alanine aminotransferase, the reasons why higher alanine aminotransferase accompanied by a lower OR in multivariate logistic regression, as inferable from our study, is that the patient has a higher transaminase on admission, which means poor liver function. For patients with poor liver function, in general, the doctor always gives patients positive liver protection treatment. Patients who have received active liver protection have recovered their liver function before surgery and can withstand the physical challenges of surgery (13,14). Jaundice has been previously reported to be patient-related risk factors, predisposing to pancreatic fistula after PD (15). The duration of jaundice is found to influence this poor outcome (16). Yeh et al. demonstrated that jaundiced patients with impaired creatinine clearance not only had a higher incidence of PJ leak, but were also more liable to experience sepsis and intraabdominal bleeding, which uniformly elicited a grave clinical course. Moreover, the cause of jaundice is the concentration of total bilirubin in the blood. With regard to the pre-existing comorbidity of respiratory diseases, it has already been widely accepted as a significant risk factor that predisposes patients to complications (17,18). In addition, the larger the diameter of the tumor, the greater the probability that the tumor will invade the surrounding blood vessels, and the higher the chance of the patient's combined vascular resection and the more intraoperative blood loss (8,19,20). Moreover, intraoperative blood loss and combined vascular resection had been proved to be risk factors for post-PD complications. In addition, the more the patient's nutritional status declines sharply; and the nutritional status of the patient directly affects the patient’s postoperative recovery. Furthermore, the smaller the inner diameter of the pancreatic duct, the more likely it is to cause pancreatic fistula, which has been confirmed by most scholars (19-22). Finally, an explanation for why we included INR-PT (international normalized ratio of prothrombin time) as a risk factor in our formula, although its P value (P=0.058) is greater than 0.05, is warranted.
Firstly, in the process of logistic regression and linear regression, the program automatically retains the INR-PT, which means that the program understands INR-PT as one of the risk factors for postoperative complications. Secondly, although its P value is greater than 0.05 yet still close to 0.05, there is still a strong correlation between INR and postoperative complications; many clinical studies have proved that coagulation function will also affect postoperative complications. P value greater than 0.05 maybe caused by insufficient sample size. On the other hand, the reasons why higher INR accompanied by a lower OR in multivariate logistic regression is that, as our study finds, patients with poor preoperative coagulation function will undergo a period of improved coagulation therapy before surgery. The worse the coagulation function patients were, the greater the intensity of the treatment they accepted, which reduces the occurrence of postoperative gastrointestinal and intraabdominal bleeding.
Through the validation of the ROC curve, the PD-POSSUM scoring system (AUC =0.689, P=0.000, 95% CI: 0.616–0.762) in our study has a larger AUC than the traditional POSSUM scoring system (AUC =0.545, P=0.271, 95% CI: 0.465–0.625) (Table 6, Figure 1), which means that the PD-POSSUM scoring system is more predictive of PD postoperative complications than traditional POSSUM scoring system. The total PD-POSSUM score of 201 patients included in this study was 92, and mean PD-POSSUM score was 0.458, which means that the average postoperative complication rate of 201 patients in this study is 45.8%.
The traditional POSSUM scoring system and p-POSSUM scoring system are based on the perioperative data of all surgical patients to predict the incidence and mortality of postoperative complications. They can be applied to various types of surgery but not specific to a certain surgery. The PD-POSSUM scoring system in our study, by collecting the perioperative data of patients undergoing pancreatoduodenectomy, creates a more suitable tool for predicting postoperative complications inpatients after pancreatoduodenectomy. Compared with the traditional POSSUM scoring system and p-POSSUM scoring system, the prediction tool is more specific and accurate for patients undergoing pancreatoduodenectomy. The fact that the PD-POSSUM scoring system has a larger AUC in the ROC curve also demonstrates this fact. And we have elucidated details in the discussion above.
This study has several limitations. First, the most obvious is the study design, which is retrospective, not a prospective, randomized control study. As a result, there may be some bias in several areas. The second limitation is that the data in this study are based on the experience of a single institution. This may lead to some deviation in the preoperative treatment of patients, such as surgical operation or drainage tube treatment. To summarize, this study has several potential limitations, and the results have not been confirmed. In the future, these results should be verified and improved in multi-agency, prospective, randomized and controlled trials and some of the above criteria should be considered.
Conclusions
In summary, body mass index, the pre-existing comorbidity of respiratory diseases, active liver function protection, total bilirubin level, diameter of pancreatic duct, tumor diameter, and international normalized ratio of prothrombin time are associated with post-PD complications. On the basis of our findings, we have made improvements to the formula of POSSUM scoring system that can help estimate and predict more precisely whether complications will actually take place. We have established a practical score (scorePD-POSSUM) that might provide individual predictions on post-PD complications. ScorePD-POSSUM can help surgeons to obtain individualized predictions of possible morbidity associated with the proposed surgery and educate patients about their appropriate expectations for the post-operative process. More importantly, prevention and reduction of complications can be prevented by early intervention.
Table S1
| Physiological score |
| Age |
| Cardiac signs |
| Respiratory signs |
| Electrocardiography |
| Systolic blood pressure (mmHg) |
| Pulse |
| Haemoglobin (g/100 mL) |
| White cell count (×1012) |
| Urea (mmol/L) |
| Sodium (mmol/L) |
| Potassium (mmol/L) |
| Glasgow coma score |
| Operative score |
| Operative severity |
| Multiple procedures |
| Total blood loss (mL) |
| Peritoneal soiling |
| Malignant disease status |
| Mode of surgery |
Table S2
| Variables | Score | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 8 | |
| Physiological factors | |||||
| Age | ≤60 | 61–70 | – | ≥85 | |
| Cardiac signs | No heart failure | Taking cardiotonic; diuretic; antihypertensive drugs | – | Peripheral edema; taking warfarin; critical increased heart | Increased jugular venous pressure; increased heart |
| Respiratory signs | No shortness of breath | Shortness of breath during exercise | – | Moderate restrictive dyspnea | Difficulty breathing at rest; pulmonary fibrosis; lung consolidation |
| Electrocardiography | Normal | – | – | Atrial fibrillation | Abnormal heart rhythm; premature beat >5/min; Q wave or ST/T segment abnormality |
| Systolic blood pressure (mmHg) | 110–130 | 100–109 or 131–170 | – | 90–99 or ≥171 | <89 |
| Pulse (time/min) | 50–80 | 81–100 or 40–49 | – | 101–120 | >120 or ≤39 |
| Haemoglobin (g/100 mL) | 130–160 | 115–129 or 161–170 | – | 101–114 or 171–180 | ≤100 or ≥181 |
| White cell count (×1012) | 4–10 | 3.1–4.0 or 10.1–20.0 | ≤3.0 or ≥20.1 | – | |
| Urea (mmol/L) | ≤7.5 | 7.6–10.1 | 10.1–15.0 | ≥15.1 | |
| Sodium (mmol/L) | >136 | 131–135 | 126–130 | <125 | |
| Potassium (mmol/L) | 3.5–5.0 | 3.2–3.4 or 5.1–5.3 | 2.9–3.1 or 5.4–5.9 | ≤2.8 or ≥6.0 | |
| Glasgow coma score | 15 | 12–14 | 9–11 | ≤8 | |
| BMI (kg/m2) | <24 | – | – | 24–28 | >28 |
| The pre-existing comorbidity of respiratory diseases | No | – | – | Yes | – |
| Normal blood coagulation function | Yes | – | No | – | – |
| Active liver function protection | Yes | No | – | – | – |
| Total bilirubin level (mmol/L) | ≤17.1 | 17.1–34.2 | 34.2–171.0 | 171.0–342.0 | >342 |
| Diameter of pancreatic duct(mm) | >5 | 3–5 | – | <3 | |
| Tumor diameter (cm) | ≤2 | – | – | >2 | |
| Total physiological score | |||||
| Operative factors | |||||
| Operative severity | Grade 1 | Grade 2 | – | Grade 3 | Grade 4 |
| Multiple procedures (time) | 1 | 2 | – | >2 | |
| Total blood loss (mL) | ≤100 | 101–500 | 501–999 | ≥1,000 | |
| Peritoneal soiling | No | Incision injury | – | Small amount of contamination and tissue necrosis | Massive contamination and tissue necrosis |
| Malignant disease status | No | Primary origin only | Lymph node metastasis | Distant metastasis | |
| Mode of surgery | Elective surgery | – | – | – | - |
| Total operative score | |||||
BMI, body mass index.
Acknowledgments
Thanks to Peng Li and Guo Peng for their valuable comments and suggestions on a previous draft of this article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-828
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-828
Availability of Data and Material: All data and material from the clinical treatment process are faithfully represented in this study and are real and available. In accordance with patient privacy protection protocol, we cannot share their information and related data.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-828). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research conformed to the Declaration of Helsinki (as revised in 2013). The present study was approved by the Ethics Committee of The Fourth Affiliated Hospital of Hebei Medical University (Shijiazhuang, China) (ID: 2016MEC078). All patients provided their written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vin Y, Sima CS, Getrajdman GI, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg 2008;207:490-8. [Crossref] [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199-210; discussion 1210-1. [Crossref] [PubMed]
- Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004;139:718-25; discussion 725-7. [Crossref] [PubMed]
- Behrman SW, Rush BT, Dilawari RA. A modern analysis of morbidity after pancreatic resection. Am Surg 2004;70:675-82; discussion 682-3. [PubMed]
- Watanabe M, Miyata H, Gotoh M, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg 2014;260:1034-9. [Crossref] [PubMed]
- Kobayashi H, Miyata H, Gotoh M, et al. Risk model for right hemicolectomy based on 19,070 Japanese patients in the National Clinical Database. J Gastroenterol 2014;49:1047-55. [Crossref] [PubMed]
- Matsubara N, Miyata H, Gotoh M, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum 2014;57:1075-81. [Crossref] [PubMed]
- Aoki S, Miyata H, Konno H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci 2017;24:243-51. [Crossref] [PubMed]
- Liang X, Shi LG, Hao J, et al. Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int 2017;16:537-44. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- El Nakeeb A, Hamed H, Shehta A, et al. Impact of obesity on surgical outcomes post-pancreaticoduodenectomy: a case-control study. Int J Surg 2014;12:488-93. [Crossref] [PubMed]
- Gaujoux S, Torres J, Olson S, et al. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol 2012;19:2908-16. [Crossref] [PubMed]
- Cramer JD, Patel UA, Samant S, et al. Liver disease in patients undergoing head and neck surgery: Incidence and risk for postoperative complications. Laryngoscope 2017;127:102-9. [Crossref] [PubMed]
- Kubota K, Aoki T, Kumamaru H, et al. Use of the National Clinical Database to evaluate the association between preoperative liver function and postoperative complications among patients undergoing hepatectomy. J Hepatobiliary Pancreat Sci 2019;26:331-40. [Crossref] [PubMed]
- Reyna-Sepúlveda F, Muñoz-Maldonado G, Pérez-Rodríguez E, et al. Prognostic factors for survival and surgical complications in Whipple's pancreatoduodenectomy during a 10-year experience. Cir Cir 2019;87:205-10. [Crossref] [PubMed]
- Yeh TS, Jan YY, Jeng LB, et al. Pancreaticojejunal anastomotic leak after pancreaticoduodenectomy--multivariate analysis of perioperative risk factors. J Surg Res 1997;67:119-25. [Crossref] [PubMed]
- India State-Level Disease Burden Initiative CRD Collaborators. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health 2018;6:e1363-74. [Crossref] [PubMed]
- Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model de-rived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773-80. [Crossref] [PubMed]
- Li Y, Zhou F, Zhu DM, et al. Novel risk scoring system for prediction of pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol 2019;25:2650-64. [Crossref] [PubMed]
- Zhao N, Cui J, Yang Z, et al. Natural history and therapeutic strategies of post-pancreatoduodenectomy abdominal fluid collections: Ten-year experience in a single institution. Medicine (Baltimore) 2019;98:e15792. [Crossref] [PubMed]
- Wang H, Shao Z, Guo SW, et al. Zhonghua Wai Ke Za Zhi 2019;57:534-9. [Analysis of prognostic factors for hyperamylasemia following pancreaticoduodenectomy]. [PubMed]
- Ohgi K, Okamura Y, Sugiura T, et al. Pancreatic attenuation on computed tomography predicts pancreatic fistula after pancreaticoduodenectomy. HPB (Oxford) 2020;22:67-74. [Crossref] [PubMed]


