
The expression of Fibulin-3 in ovarian cancer and its relationship with prognosis
Introduction
Ovarian cancer (OC) is a common malignant tumor in gynecology, and its morbidity and mortality rank among the highest among all forms of female-related cancer (1). Although great progress has been made in the development of therapeutic strategies for treating OC, most patients are diagnosed at an advanced stage because of the lack of effective early diagnostic methods, resulting in a poor 5-year survival rate of 25–30% (2). Tumor recurrence and metastasis are thought to be responsible for the poor clinical outcomes of OC. Fibulin-3 (also known as epidermal growth factor-containing fibulin-like extracellular matrix protein 1/EFEMP1) is a member of the fibulin glycoprotein family. Fibulin-3 functions mainly to maintain the stability of the basement membrane and stabilize the integrity of the extracellular matrix structure (3,4). Many studies have shown that Fibulin-3 is closely related to the development of various tumors. In pancreatic cancer (5), cervical cancer (6), glioblastoma (7), and osteosarcoma (8), high expression of Fibulin-3 suggests a poor prognosis. However, in nasopharyngeal carcinoma (9), breast cancer (10), non-small cell lung carcinoma (11), and endometrial cancer (12), Fibulin-3 was found to have potential tumor-suppressive effects. Thus, Fibulin-3 has shown varying effects in different tumor types.
Epithelial-mesenchymal transition (EMT) is a developmental process that is critical for cell differentiation, morphogenesis, and development. During this process, the epithelial cell morphology changes, cell-cell adhesion is disrupted, and polarity is lost. The characteristics of interstitial cells, particularly low adhesion, migration, invasiveness, and dissemination have become the basis of tumor cell invasion and metastasis (13). E-cadherin, Snail, and N-cadherin are important markers of EMT, and the deletion of E-cadherin expression is considered as an important marker of EMT (14,15).This study was to investigate the expression of Fibulin-3 in OC tissues, detect the expression of EMT-associated markers, E-cadherin, N-cadherin, and Snail in OC, explore the relationship between Fibulin-3 and E-cadherin, N-cadherin, and Snail as well as their relationship with OC invasion, metastasis, and prognosis. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-984).
Methods
Case data
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Chongqing Medical University (NO:2020-425) and informed consent was taken from all the patients. A total of 102 ovarian tissue specimens were used in this study, including 10 specimens of benign ovarian tumor, 8 specimens of borderline tumor, and 84 specimens of malignant tumors (66 cases of serous carcinoma, 6 cases of mucinous carcinoma, 5 cases of endometrial carcinoma, and 7 cases of clear cell carcinoma). The patients were not administered radiotherapy, chemotherapy, or other anti-tumor treatment before surgery. Samples were collected from patients admitted to the Department of Clinical Pathology of the First Affiliated Hospital of Chongqing Medical University from 2012 to 2014. Complete clinical, pathological, and follow-up data were available for all patients, and the selected cases were followed until the patient died or until the end of the study in May 2019. The follow-up time was 6–60 months and patient ages ranged between 38 and 72 years. According to the International Federation of Gynecology and Obstetrics (FIGO) classifications for staging OC, there were 37 cases of low-stage tumors (FIGO I + II) and 47 cases of high-stage tumors (FIGO III + IV). Based on the Shih and Kurman dualistic model (16), the cases were divided into: 23 cases of type I OC, 61 cases of type II OC. None of the patients received radiotherapy, chemotherapy, and other anti-tumor treatment before surgery. The pathological sections of the patients were reviewed, and archived surgical specimen paraffin blocks from the patients were used for sectioning.
Reagents
Rabbit anti-human Fibulin-3 polyclonal antibody and rabbit anti-human Snail polyclonal antibody were purchased from Bioss (Woburn, MA, USA). Rabbit anti-human E-cadherin polyclonal antibody and rabbit anti-human E-cadherin polyclonal antibody were purchased from BOSTER (Wuhan, China) A Max Vision 3 kit and the DAB chromogenic kit were purchased from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou, China).
Experimental methods
All tissue specimens were fixed in 4% neutral formalin solution. The paraffin-embedded tissues were continuously sliced into thin sections of 4 µm and dried, followed by immersion in xylene solution and gradient ethanol solution to dewax and rehydrate the samples to obtain the immunological tissues. Staining was carried out according to the manufacturer’s instructions provided in the kit. Cervical cancer tissue sections (Fibulin-3-positive) were used as positive control (6), and phosphate-buffered saline was used as the blank control.
Evaluation of results
Fibulin-3, E-cadherin, and N-cadherin were positive for brownish yellow particles in the cell membrane and cytoplasm, and Snail was positive for brownish yellow particles in the nucleus and cytoplasm. Protein expression levels were determined by the comprehensive staining intensity and percentage of positive cells. (I) The staining intensity was scored as 0–3 points: no coloring or non-staining, 0 points; light yellow, 1 point; yellow, 2 points; brown yellow, 3 points; (II) percentage of positive cells: no positive cells, 0 points; 1–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points; >75%, 4 points. The slice score was calculated as the staining intensity score plus the percentage of positive cells. Points ranging from 0 to 3 were considered as low expression, whereas >3 points was considered as high expression. Finally, two pathologists evaluated the results of immunohistochemical staining in an independent and double-blind manner.
Statistics
Data sorting, screening, and statistical analysis were performed using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). The count data were recorded as N (%) form, and the two sets of count data were analyzed using the chi-square test (with accurate probability method if necessary) to compare groups. Spearman correlation was used. An analytical method was used for correlation analysis between Fibulin-3 and other indicators. The Cox proportional hazard regression model was used to define the potential prognostic significance of individual parameters. The survival curve was drawn using GraphPad Prism software (GraphPad, Inc., La Jolla, CA, USA). Difference were considered as statistically significant at P<0.05.
Results
Expression of Fibulin-3 in human ovarian tissue specimens and its clinicopathological factors
To evaluate the protein expression of Fibulin-3 in ovarian tissues, we obtained 102 human ovarian tissue samples. According to immunohistochemical staining, Fibulin-3 was mainly concentrated in the cell membrane and cytoplasm of OC tissues (Figure 1). Fibulin-3 expression was significantly higher in OC samples than that in benign ovarian tumor and benign ovarian tumors (Figure 1). To evaluate antibody specificity, the negative control and positive control were examined and the results are shown in Figure 2. As shown in Table 1, high expression of Fibulin-3 protein in OC was observed in 45.24% of samples (38/84), while those in benign ovarian tumors was 20% (2/10) and in borderline ovarian tumor was 0% (0/8). The difference between the three groups was significant (P<0.05). At more advanced clinical FIGO stages of OC, Fibulin-3expression was significantly higher (P<0.05). There were no significant differences among the different pathology types, age, grade types, or preoperative CA125 of patients with OC (P>0.05).
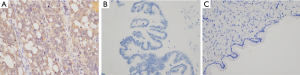
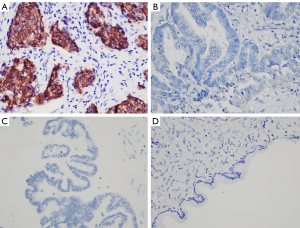
Table 1
| Characteristics | N | % | Fibulin-3 | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | ||||||||
| N | % | N | % | ||||||
| Age (years) | 0.714 | 0.398 | |||||||
| ≤45 | 22 | 26.19 | 14 | 63.64 | 8 | 36.36 | |||
| >45 | 62 | 74.81 | 33 | 53.23 | 29 | 46.77 | |||
| Type of organization | 10.926 | 0.004** | |||||||
| benign | 10 | 9.80 | 8 | 80.00 | 2 | 20.00 | |||
| borderline | 8 | 7.84 | 8 | 100.00 | 0 | 0.00 | |||
| Carcinoma | 84 | 82.35 | 46 | 54.76 | 38 | 45.24 | |||
| Pathology type | 1.313 | 0.726 | |||||||
| clear cell carcinoma | 7 | 8.33 | 5 | 71.43 | 2 | 28.57 | |||
| endometrial carcinoma | 5 | 5.95 | 2 | 40.00 | 3 | 60.00 | |||
| serous carcinoma | 66 | 78.57 | 36 | 54.55 | 30 | 45.45 | |||
| mucinous carcinoma | 6 | 7.14 | 3 | 50.00 | 3 | 50.00 | |||
| Grade type | 0.086 | 0.770 | |||||||
| type I | 23 | 27.38 | 12 | 52.17 | 11 | 47.83 | |||
| type II | 61 | 72.62 | 34 | 55.74 | 27 | 44.26 | |||
| FIGO stage | 4.377 | 0.036** | |||||||
| I + II | 37 | 44.05 | 25 | 67.57 | 12 | 32.43 | |||
| III+ IV | 47 | 55.95 | 21 | 44.68 | 26 | 55.32 | |||
| CA125 | 1.764 | 0.184 | |||||||
| <1,000 U/mL | 55 | 65.48 | 33 | 60.00 | 22 | 40.00 | |||
| >1,000 U/mL | 29 | 34.52 | 13 | 44.83 | 16 | 55.17 | |||
**, P<0.05.
Correlation between the expression intensity of Fibulin-3, Snail, E-cadherin, and N-cadherin in OC tissues
Activation of EMT program promotes tumor cell invasion and metastasis. To further determine the correlations between Fibulin-3 and EMT makers (Snail, E-cadherin, N-cadherin), we performed immunohistochemical analysis of OC (Figure 3). According to Spearman correlation analysis, there was a negative correlation between Fibulin-3 and E-cadherin expression in OC (r=‒0.252, P=0.021). Fibulin-3 was positively correlated with Snail and N-cadherin expression in OC (r=0.644, P<0.001; r=0.451, P<0.001) (Table 2).
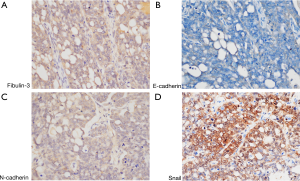
Table 2
| Fibulin-3 | r | P value | ||
|---|---|---|---|---|
| Low | High | |||
| E-cadherin | ||||
| Low | 12 | 22 | 0.252 | 0.021* |
| High | 34 | 16 | ||
| Snail | ||||
| Low | 42 | 14 | 0.644 | <0.001* |
| High | 4 | 24 | ||
| N-cadherin | ||||
| Low | 39 | 17 | 0.451 | <0.001* |
| High | 7 | 21 | ||
*, P<0.005; r relative coefficient. OC, ovarian cancer.
Univariate and multivariate analysis of prognostic variables in OC
The factors of age (≤45 and >45 years), pathology type (serous carcinoma, mucinous carcinoma, endometrial carcinoma, clear cell carcinoma), grade type (type I, type II), FIGO stage (I + II, III + IV), and Fibulin-3 expression (low, high) in patients with OC were analyzed by Cox analysis. Univariate Cox regression analyses revealed that FIGO stage (III + IV; HR: 3.913, 95% CI: 1.702–8.999, P=0.001) and Fibulin-3 expression (high; HR: 11.321, 95% CI: 4.346–29.491, P<0.001) were significantly associated with survival. However, no significant associations were revealed between age (≥45; P=0.172), pathology type (P=0.158), or grade type (P=0.794). Multivariate Cox regression analysis showed that Fibulin-3 (HR: 9.225, 95% CI: 3.484–24.426, P<0.001) was an independent prognostic maker for survival (Table 3). In contrast, FIGO stage was not an independent prognostic factor (P=0.067).
Table 3
| Characteristics | Univariate Cox | Multivariate Cox | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Age (years) | 1.849 | 0.765–4.466 | 0.172 | 1.503 | 0.610–3.701 | 0.376 | |
| Pathology type | 0.710 | 0.441–1.142 | 0.158 | 0.871 | 0.495–1.532 | 0.632 | |
| Grade type | 1.107 | 0.516–2.371 | 0.794 | 0.935 | 0.407–2.149 | 0.874 | |
| FIGO stage | 3.913 | 1.702–8.999 | 0.001* | 2.378 | 0.941–6.012 | 0.067 | |
| Fibulin-3 | 11.321 | 4.346–29.491 | <0.001* | 9.225 | 3.484–24.426 | <0.001* | |
*, P<0.005.
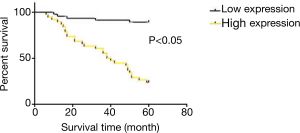
Survival analysis
The results of survival curve analysis are shown in Figure 4. The average 5-year survival rate of this group was 58.3%. Survival analysis using GraphPad Prism showed that survival rates of patients with high and low expression survival of Fibulin-3 were 23.6% and 89.1%, respectively, revealing a significant difference (P>0.05).
Discussion
Invasion and metastasis are the main causes of death in 90% of patients with OC. Fibulin-3 is a member of the fibulin family of proteins (17) and has a highly homologous structure with a wide range of expression. Fibulin is mainly found in the basement membrane, extracellular matrix, and other parts of cells (18); it is involved in the interaction between cells and the matrix, can act on endothelial cells, and participates in body repair (19). Our immunohistochemical analysis revealed high expression of Fibulin-3 in OC tissues as compared to in benign ovarian tumor and borderline ovarian tumor tissues. This high expression of Fibulin-3 in OC is similar to the findings of previous studies of pancreatic adenocarcinoma, cervical cancer, colon carcinoma, and glioma. In this study, the expression level of Fibulin-3 was found to be associated with the FIGO stage of epithelial OC. Expression rates of 32.43% and 55.32% were observed for FIGO stage I–II and III–IV OC,, respectively. Additionally, Fibulin-3 expression was found to be related to poor prognosis. For patients with high expression of Fibulin-3, the 5-year survival rate was lower than that of patients with low expression. Similar outcomes were reported by Chen et al. (20), who demonstrated an association between high expression of Fibulin-3 and poor survival of patients with OC. Furthermore, we observed increased expression from normal to benign to cancer in a larger series of samples. Our results support that Fibulin-3 facilitated OC cell development and metastasis. Additionally, our findings demonstrate an association of Fibulin-3 expression with EMT markers (E-cadherin, Snail, and N-cadherin).
EMT has recently become a hot spot in the field of cancer research. EMT refers to the phenotype obtained by mesenchymal cells after epithelial cells undergo various biochemical changes (21). E-cadherin is a tumor suppressor gene; numerous studies have shown that inhibition of E-cadherin function or expression can cause cell morphology to transform into stromal cells resulting in cell migration, invasion, and metastasis (22-24). Some studies have shown that E-cadherin expression is gradually down-regulated in poorly differentiated serous carcinomas transformed from borderline serous tumors (25,26). N-cadherin, a mesenchymal marker of EMT, is also involved in the invasion and metastasis of tumors. The Snail family of transcription factors trigger EMT by inhibiting E-cadherin (27). Snail is the earliest known transcription repressor of E-cadherin expression in EMT, and its pathological activation allows tumor cells to acquire invasive characteristics. Fibulin-3 significantly affects the expression of several key factors in EMT. In some cancer cell lines, an interaction mechanism of Fibulin-3 and EMT has been suggested (6,8). In pancreatic adenocarcinomas, Fibulin-3 binds EGFR, causing autophosphorylation of EGFR at Tyr-992 and Tyr-1068 and subsequent phosphorylation of AKT at Thr-308 and ERK at Thr-202 and Tyr-204, thus accelerating the growth of pancreatic adenocarcinoma (5). In colon carcinoma, Fibulin-3 promotes cancer cell growth, migration, and invasion through a mechanism correlated with p38α and p38β activation (28). Fibulin-3 has been shown to facilitate cervical cancer cell development and metastasis by activating the PI3K-Akt-mTOR signal transduction pathway (6). In malignant gliomas, Fibulin-3 secreted by glioma cells activated DLL4-Notch signaling to induce proangiogenic behavior and promote glioma cell motility and invasion (29). In the present study, according to Spearman correlation analysis, when Fibulin-3 was overexpressed, E-cadherin was down-regulated, whereas N-cadherin and Snail were up-regulated. Fibulin-3 was negatively correlated with E-cadherin expression and positively correlated with the expression of Snail and N-cadherin expression. Finally, in multivariate analysis using Cox proportional hazard models, Fibulin-3 expression appeared to be an independent unfavorable prognostic factor. Based on our findings and those of previous studies, the Fibulin-3 pathway may be involved in EMT in OC, thereby causing cancer cell metastasis (30). High Fibulin-3 expression was found to be associated with poor prognosis in OC.
Conclusions
Fibulin-3 protein may play an important role in the occurrence and development of OC. Our results indicate that Fibulin-3 is overexpressed in OC and may promote tumor invasion and metastasis through EMT. Thus, Fibulin-3 may be useful as an independent predictive factor of poor prognosis of patients with OC. Further studies should be performed to evaluate whether Fibulin-3 can be used as a target for the clinical treatment of OC.
Acknowledgments
We would like to thank Key Laboratory of Life science (Chongqing, China) and Department of Pathophysiology for guidance and partial sponsorship.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-984
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-984
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-984
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-984). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Chongqing Medical University (NO: 2020-425) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Januchowski R, Zawierucha P, Ruciński M, et al. Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. Biomed Res Int 2014;2014:365867. [Crossref] [PubMed]
- Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat. Rev Cancer 2009;9:415-28. [Crossref] [PubMed]
- de Vega S, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell. Mol. Life Sci 2009;66:1890-902. [Crossref] [PubMed]
- Gallagher WM, Currid CA, Whelan LC. Fibulins and cancer: friend or foe? Trends Mol Med 2005;11:336-40. [Crossref] [PubMed]
- Seeliger H, Camaj P, Ischenko I, et al. EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol Cancer Res 2009;7:189-98. [Crossref] [PubMed]
- Li J, Qi C, Liu X, et al. Fibulin-3 knockdown inhibits cervical cancer cell growth and metastasis in vitro and in vivo. Sci Rep 2018;8:10594. [Crossref] [PubMed]
- Hu B, Thirtamara-Rajamani KK, Sim H, et al. Fibulin-3 is uniquely upregulated in malignant gliomas and promotes tumor cell motility and invasion. Mol Cancer Res 2009;7:1756-70. [Crossref] [PubMed]
- Wang S, Zhang D, Han S, et al. Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway. Sci Rep 2017;7:6215. [Crossref] [PubMed]
- Hwang CF, Chien CY, Huang SC, et al. Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol 2010;222:367-79. [Crossref] [PubMed]
- Sadr-Nabavi A, Ramser J, Volkmann J, et al. Decreased expression of angiogenesis antagonist EFEMP1 in sporadic breast cancer is caused by aberrant promoter methylation and points to an impact of EFEMP1 as molecular biomarker. Int J Cancer 2009;124:1727-35. [Crossref] [PubMed]
- Kim EJ, Lee SY, Woo MK, et al. Fibulin-3 promoter methylation alters the invasive behavior of non-small cell lung cancer cell lines via MMP-7 and MMP-2 regulation. Int J Oncol 2012;40:402-8. [PubMed]
- Yang T, Qiu H, Bao W, et al. Epigenetic inactivation of EFEMP1 is associated with tumor suppressive function in endometrial carcinoma. PLoS One 2013;8:e67458. [Crossref] [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740-6. [Crossref] [PubMed]
- Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol 2009;174:1588-93. [Crossref] [PubMed]
- Kang Y, Joan M. Epithelial-Mesenchymal Transitions Twist in Development and Metastasis. Cell 2004;118:277-9. [Crossref] [PubMed]
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433-43. [Crossref] [PubMed]
- Timpl R, Sasaki T, Kostka G, et al. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 2003;4:479-89. [Crossref] [PubMed]
- Henrotin Y, Gharbi M, Mazzucchelli G, et al. Fibulin 3 peptides Fib3-1 and Fib3-2 are potential biomarkers of osteoarthritis. Arthritis Rheum 2012;64:2260-7. [Crossref] [PubMed]
- Kobayashi N, Kostka G, Garbe JH, et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem 2007;282:11805-16. [Crossref] [PubMed]
- Chen J, Wei D, Zhao Y, et al. Overexpression of EFEMP1 correlates with tumor progression and poor prognosis in human ovarian carcinoma. PLoS One 2013;8:e78783. [Crossref] [PubMed]
- Chen T, You Y, Jiang H, et al. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol 2017;232:3261-72. [Crossref] [PubMed]
- Vleminckx K, Vakaet L, Mareel M, et al. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991;66:107-19. [Crossref] [PubMed]
- Perl AK, Wilgenbus P, Dahl U, et al. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998;392:190-3. [Crossref] [PubMed]
- Mareel MM, Behrens J, Birchmeier W, et al. Down-regulation of E-cadherin expression in Madin Darby canine kidney (MDCK) cells inside tumors of nude mice. Int J Cancer 1991;47:922-8. [Crossref] [PubMed]
- Cheng JC, Auersperg N, Leung PC. Inhibition of p53 represses E-cadherin expression by increasing DNA methyltransferase-1 and promoter methylation in serous borderline ovarian tumor cells. Oncogene 2011;30:3930-42. [Crossref] [PubMed]
- Cheng JC, Auersperg N, Leung PC. Inhibition of p53 induces invasion of serous borderline ovarian tumor cells by accentuating PI3K/Akt-mediated suppression of E-cadherin. Oncogene 2011;30:1020-31. [Crossref] [PubMed]
- Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000;2:76-83. [Crossref] [PubMed]
- Arechederra M, Priego N, Vázquez-Carballo A, et al. p38 MAPK down-regulates fibulin 3 expression through methylation of gene regulatory sequences: role in migration and invasion. J Biol Chem 2015;290:4383-97. [Crossref] [PubMed]
- Nandhu MS, Hu B, Cole SE, et al. Novel paracrine modulation of Notch-DLL4 signaling by fibulin-3 promotes angiogenesis in high-grade gliomas. Cancer Res 2014;74:5435-48. [Crossref] [PubMed]
- Huang RY, Chung VY, Thiery JP. Targeting pathways contributing to epithelial-mesenchymal transition (EMT) in epithelial ovarian cancer. Curr Drug Targets 2012;13:1649-53. [Crossref] [PubMed]


