Association between BRAF mutant classification and the efficacy of pemetrexed-based chemotherapy in Chinese advanced non-small cell lung cancer patients: a multicenter retrospective study
Introduction
Lung cancer has always been the leading cause of cancer death in all populations worldwide (1). Conventional chemotherapies used to be the cornerstone for treatment in advanced non-small cell lung cancer (NSCLC) cancer patients (2). During the past three decades, the discovery of driver genes and target therapies have significantly improved the outcome in oncogenic-addictive NSCLC patients (3,4). BRAF was proto-oncogene encoding a serine/threonine-protein kinase which promotes cell proliferation and survival (5). BRAF mutations are commonly seen in melanoma (6) and papillary thyroid carcinoma (7) and also played as one of the major oncogenic drivers occurring in 2–4% NSCLC patients (5,8). BRAF mutations are mainly localized in the kinase domain and about 50–80% of them are V600E mutation (9,10). However, the prognostic value of V600E mutation remains unclear (9,11) and non-V600E mutated cases showed distinctive and complicated characteristics from V600E mutant carriers (9).
Based on the increasing knowledge of the biological activation mechanism and response of inhibitors in NSCLC patients with BRAF mutations, a functional mutation classification system has been established recently (12,13). In detail, class 1 mutants refer to the RAS-independent kinase-activating V600 monomers; class 2 mutants refer to the RAS-independent kinase-activating dimers which are resistant to vemurafenib; class 3 refer to the RAS-dependent kinase-inactivating heterodimers. Previous data showed that the advanced NSCLC patients with BRAF mutation (18 cases with V600E and 18 cases with non-V600E) had similar OS compared with patients without BRAF mutation, however, V600E-mutated patients tended to have shorter PFS after platinum-based chemotherapy compared with non-V600E-mutated patients (4.1 vs. 8.9 months; P=0.297) (14). Increasing data support the recommendation of BRAF and downstream pathway targeted therapy in advanced NSCLC patients with V600E mutation (15). However, the prognosis and treatment recommendation for patients with non-BRAF V600 mutations remains controversial (16,17). BRAF inhibitor in the combination of downstream targets such as MEK inhibitor may be better than monotherapy by reducing the drug-resistance in patients with non-BRAF V600 mutations (18).
Although target therapy should be the promising first-line treatment for patients with BRAF mutations, most of them still have to accept chemotherapy in conditions of inaccessible to target agents or progression from target therapy (19). Besides, the advance of molecular testing method could help us to explore more non-V600E mutations which would potentially contribute to the biological development of disease (20). Recently, results from one large cohort study from France showed that no clear evidence supports the influence of BRAF status in outcomes of patients treated with chemotherapy and patients treated by the first-line taxane had the poorest PFS (19). In this study, we aimed to explore the clinical value of the functional classification of BRAF mutations and optimizing the selection of chemotherapy in this cohort study. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-480).
Methods
Patients
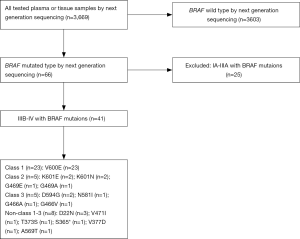
We conducted a multicenter, retrospective study involving several hospitals in China between May 2014 and May 2019. The genetic alterations results obtained from 3,669 plasma or tissue samples of NSCLC patients who underwent the next-generation sequencing (NGS) detection, and IIIB-IV with BRAF mutations were 41 cases. Histology subtyping was determined according to the 2015 World Health Organization classification. Tumor stage was based on the 7th edition of the Lung Cancer Staging system from the American Joint Committee on Cancer. Age, smoking status, East Cooperative Oncology Group performance status, histology, disease stage, brain or bone metastasis, and molecular information were documented at first diagnosis. All clinical information, including diagnosis, treatment, and clinical outcome, was collected through the system and confirmed by local professional oncologists. Patients were followed from the date of diagnosis until the date of death from all causes or last approachable follow-up. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) (21). The treatment response was evaluated by CT scans at the baseline of initial therapy and every 6 weeks thereafter. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Chinese Academy of Sciences University Cancer Hospital (Zhejiang Cancer Hospital) Ethics Committee (No.: IRB-2020-188) and written informed consent was obtained from each patient to use the clinical data for research before the medical intervention started.
Molecular detection
Targeted region capture combined NGS was performed for the 41 NSCLC patients. Genomic DNA sequencing libraries were prepared using the protocols recommended by the TruSeq DNA Library Preparation Kit (Illumina, San Diego, CA, USA). For samples close to the minimum input requirement, additional pre-capture polymerase chain reaction cycles were performed to generate sufficient product for hybridization. The libraries were hybridized to custom-designed probes (Integrated DNA Technology, Coralville, IA, USA) including all exons of 170 genes and selected introns of anaplastic lymphoma kinase, RET, and ROS1 for the detection of genomic rearrangements. DNA sequencing was performed on a HiSeq3000 sequencing system (Illumina) with 2×75 bp paired-end reads. The reads were aligned to the human genome build GRCh37 using BWA (a Burrows-Wheeler aligner). Somatic single nucleotide variant and indel calls were generated using MuTect and GATK, respectively. Somatic copy number alterations were identified with CONTRA. Genomic rearrangements were identified by software developed in-house to analyze chimeric read pairs.
Statistical analyses
Kaplan-Meier curves and the two-sided log-rank test were used for univariate survival analyses. The Cox proportional hazards model was used to complete the uni- and multivariate survival analyses with the hazard ratio (HR) and corresponding 95% confidence interval. Progression-free survival (PFS) was defined as the time from the date of initial treatment to the date of systemic progression or death or censored at the date of last follow up, whichever came first to trigger the event. Significance between groups was defined as P values <0.05. Statistical analyses were performed using the R software/environment (URL http://www.R-project.org).
Results
Clinicopathologic characteristics
We identified 66 NSCLC patients with BRAF mutation from 3,669 NSCLC patients, and 41 patients with advanced NSCLC with BRAF mutation were enrolled in this study (Figure 1). There were 36 males and 5 females, with a median age of 69 years (range, 34–83 years). The smoking history was divided into former or current (n=4) and never (n=37).
BRAF mutations
Forty-one patients with IIIB/IV stages of NSCLC had BRAF mutations. The BRAF mutants in this study were classified into four groups according to reference (22), included class 1 [n=23 (56.1%)], class 2 [n=5 (12.2%)], class 3 [n=5 (12.2%)], and others [n=8 (19.5%)] (Table 1, Figure 2). Eight patients were stage IIIB and 33 were stage IV according to the IASLC classification of lung adenocarcinoma. The detailed characteristics are listed in Table 2. Collectively, the frequency of BRAF mutants included 97.6% (40/41) point mutations and 2.4% (1/41) missense mutation. The concurrent oncogenic alterations included EGFR (n=3), KRAS (n=1), EML-ALK (n=1) (Figure 2). The baseline clinic-genetic characteristics and chemotherapy in all patients and comparison among the four groups of patients were shown in Tables 2 and 3, respectively. No significant difference existed concerning age, gender, ECOG PS, clinical stage at diagnosis, smoking status, brain and bone metastasis, concurrent oncogenic mutations, and first- and second-lines of pemetrexed-based chemotherapy among four groups (Table 3).
Table 1
| Class | BRAF mutations |
|---|---|
| Class 1 | V600E/L/D/K/M/R |
| Class 2 | P367L/S, E451Q, G464V/E/A, G469A/V/R/S, L485W, N486_A489delinK, N486_P490del, E586K, L597Q/R/S/V, T599T/S/I/K, K601E/N/T, K601_S602delinsNT, A712T, KDD, fusions |
| Class 3 | D287H, V459L, G466V/E/A, S467L, G469E, N581S/I/T, D594A/G/H/N, F595L, G596D/R |
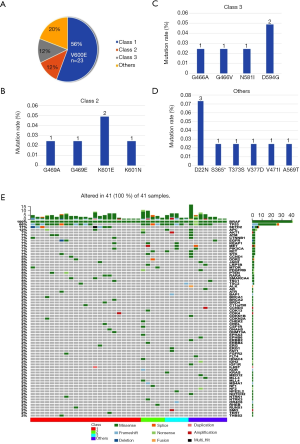
Table 2
| Characteristic | Number (%) |
|---|---|
| Gender | |
| Male | 36 (87.8) |
| Female | 5 (12.2) |
| Age (years) | |
| Mean | 66.1 |
| ≤60 | 7 (17.1) |
| >60 | 34 (82.9) |
| Smoking status | |
| No | 37 (90.2) |
| Yes | 4 (9.8) |
| Histology | |
| Adenocarcinoma | 29 (70.7) |
| Non-adenocarcinoma | 12 (29.3) |
| ECOG PS | |
| 0–1 | 33 (80.5) |
| ≥2 | 8 (19.5) |
| Clinical stage | |
| IIIB | 8 (19.5) |
| IV | 33 (80.5) |
| Brain metastases before treatment | |
| No | 38 (92.7) |
| Yes | 3 (7.3) |
| Bone metastases before treatment | |
| No | 34 (82.9) |
| Yes | 7 (17.1) |
| BRAF-mutant group | |
| Class 1 | 23 (56.1) |
| Class 2 | 5 (12.2) |
| Class 3 | 5 (12.2) |
| Others | 8 (19.5) |
| Concurrent oncogenic mutations | |
| No | 36 (87.8) |
| Yes | 5 (12.2) |
| First-line chemotherapy | |
| Pemetrexed-based | 15 (36.6) |
| Non-pemetrexed | 26 (63.4) |
| Second-line chemotherapy | |
| Pemetrexed-based | 3 (7.3) |
| Non-pemetrexed based | 38 (92.7) |
Table 3
| Characteristic | Class 1 | Class 2 | Class 3 | Others | P |
|---|---|---|---|---|---|
| Gender | 0.58 | ||||
| Male | 21 (91.3%) | 0 (0.0%) | 1 (20.0%) | 2 (25.0%) | |
| Female | 2 (8.7%) | 5 (100.0%) | 4 (80.0%) | 6 (75%) | |
| Age (years) | 0.54 | ||||
| Mean | 65.1 | 70.8 | 67.4 | 65.3 | |
| ≤60 | 5 (21.7%) | 0 (0.0%) | 0 (0.0%) | 2 (25.0%) | |
| >60 | 18 (78.3%) | 5 (100.0%) | 5 (100.0%) | 6 (75.0%) | |
| Smoking status | 0.79 | ||||
| No | 20 (87.0%) | 5 (100.0%) | 5 (100.0%) | 7 (87.5%) | |
| Yes | 3 (13.0%) | 0 (0.0%) | 0 (0.0%) | 1 (12.5%) | |
| Histology | 0.11 | ||||
| Adenocarcinoma | 16 (69.6%) | 2 (40.0%) | 3 (60.0%) | 8 (100.0%) | |
| Non-adenocarcinoma | 7 (30.4%) | 3 (60.0%) | 2 (40.0%) | 0 (0.0%) | |
| ECOG PS | 0.87 | ||||
| 0–1 | 18 (78.3%) | 4 (80.0%) | 5 (100.0%) | 6 (75.0%) | |
| ≥2 | 5 (21.7%) | 1 (20.0%) | 0 (0.0%) | 2 (25.0%) | |
| Clinical stage | 0.21 | ||||
| IIIB | 19 (82.6%) | 2 (40.0%) | 2 (40.0%) | 0 (0.0%) | |
| IV | 4 (17.4%) | 3 (60.0%) | 3 (60.0%) | 8 (100.0%) | |
| Brain metastases before treatment | 0.39 | ||||
| No | 20 (87.0%) | 5 (100.0%) | 5 (100.0%) | 8 (100.0%) | |
| Yes | 3 (13.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Bone metastases before treatment | 0.53 | ||||
| No | 18 (78.3%) | 5 (100.0%) | 5 (100.0%) | 6 (75.0%) | |
| Yes | 5 (21.7%) | 0 (0.0%) | 0 (0.0%) | 2 (25.0%) | |
| Co-occurring oncogenic alterations | 0.61 | ||||
| No | 21 (91.3%) | 5 (100%) | 4 (80.0%) | 6 (75.0%) | |
| Yes | 2 (8.7%) | 0 (0.0%) | 1 (20.0%) | 2 (25.0%) | |
| First-line chemotherapy | 0.26 | ||||
| Pemetrexed-based | 10 (43.5%) | 1 (20.0%) | 0 (0.0%) | 4 (50.0%) | |
| Non-pemetrexed | 13 (56.5%) | 4 (80.0%) | 5 (100.0%) | 4 (50.0%) | |
| Second-line chemotherapy | 0.86 | ||||
| Pemetrexed-based | 2 (8.7%) | 0 (0.0%) | 0 (0.0%) | 1 (12.5%) | |
| Non-pemetrexed based | 21 (91.3%) | 5 (100.0%) | 5 (100.0%) | 7 (87.5%) | |
First-line chemotherapy
All patients received a first-line chemotherapy regimen, including pemetrexed/platinum (n=13), pemetrexed monotherapy (n=2), paclitaxel/platinum (n=3) and gemcitabine/platinum (n=19), others (n=4). All chemotherapy regimens were calculated according to the standard dose of the NCCN guideline [pemetrexed (500 mg/m2 on day 1); gemcitabine (1,000–1,250 mg/m2 on days 1 and 8); paclitaxel (175 mg/m2 on day 1); carboplatin (AUC =5 on day 1); and cisplatin (75 mg/m2 on day 1)]. Of the 41 patients, 2 received chemotherapy in combination with bevacizumab and 3 received icotinib after first-line chemotherapy. The ORR and DCR of pemetrexed-based chemotherapy were 33.3% (5/15) and 53.3% (8/15), respectively. The ORR and DCR of the other chemotherapy regimens were 26.9% (7/26) and 42.3% (11/26), respectively. The median PFS (mPFS) for the 15 patients who received pemetrexed-based chemotherapy was 7.0 months, while the mPFS of the 26 patients who received other chemotherapy regimens was 4.0 months [P<0.001, HR =0.31 (95% CI, 0.15–0.61); Table 4, Figure 3A]. Of 23 patients with class 1 BRAF mutations, the pemetrexed-based chemotherapy-treated patients (n=10) have better mOS than non-pemetrexed-based chemotherapy-treated patients (n=13) was significantly different [30.0 vs. 22.0 months, P<0.001, HR =0.29 (95% CI, 0.12–0.73); Figure 3B]. Overall survival of patients with class 1 BRAF mutation treated with pemetrexed-based or other chemotherapy regimens as first-line chemotherapy (30 vs. 22 months, P=0.0002) (Figure 3C).
Table 4
| Covariant | Coefficient | Standard error | P value | HR | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age (≤60 vs.>60) | 0.99 | 0.57 | 0.99 | 1.01 | 3.27×10−1 | 3.00 |
| Gender (female vs. male) | 0.31 | 0.88 | 0.18 | 0.31 | 5.46×10−2 | 1.71 |
| Smoking status (no vs. yes) | 0.62 | 0.62 | 0.45 | 1.6 | 1.87×10−1 | 2.09 |
| Histology (adenocarcinoma vs. non-adenocarcinoma) | 0.01 | 0.51 | 0.99 | 0.99 | 3.73×10−1 | 2.73 |
| Clinical stage (IIIB vs. IV) | 0.73 | 0.51 | 0.54 | 1.37 | 2.67×10−1 | 2.00 |
| First-line chemotherapy (pemetrexed-based vs. non-pemetrexed) | 49.22 | 1.39 | 0.005 | 0.02 | 3.23 | 750.62 |
| Pemetrexed-based chemotherapy (no vs. yes) | 0.00 | 1.54 | 3.93×10−5 | 0.00 | 8.84×10−5 | 0.04 |
| BRAF mutant group (Class 1 vs. Class 2 vs. Class 3 vs. non-class 1–3) | 1.17 | 0.22 | 9.72×10−8 | 3.21 | 2.09 | 4.92 |
| Co-occurring oncogenic alterations (no vs. yes) | 1.88 | 0.86 | 0.46 | 0.53 | 9.87×10−2 | 2.87 |
HR, hazard ratio; CI, confidence interval.

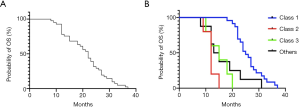
Overall survival (OS)
The median OS (mOS) of the 41 patients IIIB/IV NSCLC patients was 22.0 months (Figure 4A). A significant improvement of OS existed in patients with BRAF mutation of class 1 compared with class 2, class 3 and others (n=23.0, 5.0, 5.0, 8.0; 25.0 vs. 12.0, 15.0 and 14.0 months, P<0.0001; Figure 4B). Multivariate analysis revealed that the pemetrexed-based chemotherapy and class 1 BRAF mutated were independent prognostic factors for OS in all patients after adjusting for known characteristic factors (P<0.001; Table 4).
Discussion
We have carried out the study to explore the efficacy of pemetrexed-based chemotherapy in advanced NSCLC patients with the different functional classification of BRAF mutations. Patients who treated by first-line pemetrexed-based chemotherapy had better PFS and OS than other regimens. Furthermore, patients with class 1 BRAF mutants had a better OS than other groups after first-line pemetrexed-based chemotherapy. As far as we know, this is the first study reported the positive association between the efficacy of pemetrexed-based chemotherapy and the functional classification of BRAF mutation in advanced NSCLC patients.
BRAF mutations in NSCLC have been well-known as the unique driver-gene alterations than common mutations (23). Dagogo-Jack et al. (24) reported a significantly longer OS for BRAF-mutant NSCLC patients with class 1 than other classes after first-line chemotherapy (1 vs. 2, P<0.001; 1 vs. 2, P=0.023). However, Lin et al. (22) found comparable OS outcomes among all classes of BRAF mutation by 28.6, 13.9 and 20.2 months for classes 1, 2 and 3, respectively (P=0.585). The controversy of those results may intratumor heterogeneity of BRAF mutations and response to different regimens of chemotherapy. Nevertheless, according to the available evidence, patients with class 2 and 3 appeared to have a worse outcome than patients with class 1.
Before the BRAF mutation story initiated from melanoma to the unique subset in NSCLC (25), conventional platinum-based chemotherapy is the cornerstone treatment in advanced NSCLC patients with BRAF mutations (26). Platinum-based chemotherapies were not effective in metastatic NSCLC patients with BRAF V600E (14). Therefore, searching for better chemotherapeutic agents become very important when target therapy is inaccessible. Pemetrexed is a multitargeted antifolate and it could be used in combination with platinum in first-line or monotherapy for subsequent lines and as well as maintenance therapy (27). Over eight years survival benefit from pemetrexed treatment in an advanced NSCLC case with BRAF mutation has been reported in Japan recently (28). The higher frequency of adenocarcinoma (69.6%) in patients with class 1 BRAF mutation could also indicate the potential better response to pemetrexed based chemotherapy (29). Liang and colleagues have launched interesting research on long-term pemetrexed response in NSCLC with driver oncogenes (30). Although BRAF mutation has not included in their research, the mechanism of significantly better survival from long-term pemetrexed response in oncogenic-addiction patients should be explored.
We have also explored the concurrent driver genetic alterations in this study. The advanced NSCLC patients with BRAF mutation have seldomly co-occurrence with other driver genes (5). Under screening by the known driver-gene panel in NSCLC, only 5 patients have been detected and three of them harboring EGFR mutation have received icotinib. The other two patients with KRAS A146T and EML4-ALK respectively did not receive any target therapies. Of note, two patients with class 1 BRAF mutation co-occurring with EGFR mutation but not KRAS consistent with mutual exclusivity existed between class 1 BRAF mutation and KRAS mutation in a previous study (22).
There are some limitations to our research. Firstly, this is a retrospective study and the enrollment of patients could be biased. However, it is not easy for us to collected patients with a rare mutation from a large cohort by NGS testing. Secondly, although the standard recommendation for patients with BRAF V600E mutation should be the combination of BRAF and MEK inhibitors (31), none of the patients have ever been treated by targeted therapy due to numeric reasons. We believe that the value of our research is to pursue the most available and effective chemotherapy for those patients who could not afford those expensive targeted drugs outside of clinical trials.
Conclusions
Pemetrexed-based chemotherapy treatments were more effective than other regimens in advanced NSCLC patients with BRAF mutations. The best clinical benefit of first-line pemetrexed-based chemotherapy was observed in patients with class 1 BRAF mutants among all patients. In that case, we assumed that pemetrexed-based chemotherapy could be an alternative treatment of choice in NSCLC patients with BRAF mutation when the targeted therapy is unavailable. Besides, more research and workforce are warranted in this area.
Acknowledgments
Funding:
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-480
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-480
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-480
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-480). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Chinese Academy of Sciences University Cancer Hospital (Zhejiang Cancer Hospital) Ethics Committee (No.: IRB-2020-188) and written informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Lwin Z, Riess JW, Gandara D. The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. Journal of thoracic disease 2013;5:S556-S564. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Luk PP, Yu B, Ng CC, et al. BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res 2015;4:142-8. [PubMed]
- Edlundh-Rose E, Egyha S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 2006;16:471-8. [Crossref] [PubMed]
- Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625-7. [Crossref] [PubMed]
- Baik CS, Myall NJ, Wakelee HA. Targeting BRAF-Mutant Non-Small Cell Lung Cancer: From Molecular Profiling to Rationally Designed Therapy. Oncologist 2017;22:786-96. [Crossref] [PubMed]
- Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016;91:23-8. [Crossref] [PubMed]
- Bracht JWP, Karachaliou N, Bivona T, et al. BRAF Mutations Classes I, II, and III in NSCLC Patients Included in the SLLIP Trial: The Need for a New Pre-Clinical Treatment Rationale. Cancers (Basel) 2019;11:1381. [Crossref] [PubMed]
- Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25:138-42. [Crossref] [PubMed]
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of BRAF. Cell 2004;116:855-67. [Crossref] [PubMed]
- Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017;548:234. [Crossref] [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Noeparast A, Teugels E, Giron P, et al. Non-V600 BRAF mutations recurrently found in lung cancer predict sensitivity to the combination of Trametinib and Dabrafenib. Oncotarget 2016;8:60094-108. [Crossref] [PubMed]
- Gower A, Wang Y, Giaccone G. Oncogenic drivers, targeted therapies, and acquired resistance in non-small-cell lung cancer. J Mol Med 2014;92:697-707. [Crossref] [PubMed]
- Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 2013;31:1097. [Crossref] [PubMed]
- Couraud S, Barlesi F, Fontaine-Deraluelle C, et al. Clinical outcomes of non-small-cell lung cancer patients with BRAF mutations: results from the French Cooperative Thoracic Intergroup biomarkers France study. Eur J Cancer 2019;116:86-97. [Crossref] [PubMed]
- Tuononen K, Mäki-Nevala S, Sarhadi VK, et al. Comparison of targeted next-generation sequencing (NGS) and real-time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin‐fixed, paraffin-embedded tumor material of non-small cell lung carcinoma-superiority of NGS. Genes Chromosomes Cancer 2013;52:503-11. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Lin Q, Zhang H, Ding H, et al. The association between BRAF mutation class and clinical features in BRAF-mutant Chinese non-small cell lung cancer patients. J Transl Med 2019;17:298. [Crossref] [PubMed]
- Brustugun OT, Khattak AM, Trømborg AK, et al. BRAF-mutations in non-small cell lung cancer. Lung Cancer 2014;84:36-8. [Crossref] [PubMed]
- Dagogo-Jack I, Martinez P, Yeap BY, et al. Impact of BRAF Mutation Class on Disease Characteristics and Clinical Outcomes in BRAF-mutant Lung Cancer. Clin Cancer Res 2019;25:158. [Crossref] [PubMed]
- Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997-7000. [PubMed]
- Huang CL, Yokomise H, Fukushima M, et al. Tailor-made chemotherapy for non-small cell lung cancer patients. Future Oncol 2006;2:289-99. [Crossref] [PubMed]
- Grønberg BH, Bremnes RM, Fløtten Ø, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced Non–small-cell lung cancer. J Clin Oncol 2009;27:3217-24. [Crossref] [PubMed]
- Nakanishi Y, Nakagawa Y, Tsujino I, et al. Favorable outcome with pemetrexed treatment for advanced BRAF-V600E-positive lung adenocarcinoma in a patient followed up over 8 years. J Thorac Oncol 2018;13:e199-e202. [Crossref] [PubMed]
- Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC). J Thorac Oncol 2007;2:S851. [Crossref]
- Liang Y, Wakelee HA, Neal JW. Relationship of Driver Oncogenes to Long-Term Pemetrexed Response in Non-Small-Cell Lung Cancer. Clin Lung Cancer 2015;16:366-73. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]