Clinicopathological characteristics and survival outcomes of younger patients with gastric cancer: a systematic review and meta-analysis
Introduction
Gastric cancer is an aggressive malignancy and remains the third leading cause of cancer-related death worldwide (1,2). Although the overall incidence of gastric cancer showed a decline worldwide, younger cancer patients had increased obviously during the last decades (3). The growing incidence, as well as its aggressive biological behavior as reported (4,5), has renewed interest in the surgery-based management of younger gastric cancer patients with a focus on therapeutic strategies.
To date, the survival outcomes of younger patients were still controversial. Previous data reported that younger patients had worse survival rates than older (6-9), whereas several studies showed a similar prognosis (10-20). Some studies even expressed that younger patients were associated with improved survival outcomes (21-30). A significant reason for these inconsistent findings from published studies was the different age cutoffs on defining younger patients (6,7,29,30). A published meta-analysis has reported improved 5-year survival in the younger group. However, it was primarily limited to the small sample size and significant heterogeneity (31). Besides, there was currently no randomized clinical trial that targeted the issue.
As such, our study aimed to compare the clinicopathological characteristics, postoperative complications, as well as survival outcomes between younger and older patients with gastric cancer through systematic review and meta-analysis, thus providing evidence for the development of guiding strategies for younger gastric cancer patients. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2024).
Methods
Search strategy
Clinical studies were systematically searched from PubMed, Web of Science, Embase, and The Cochrane Library. The following fields were used for the search: “gastric” or “stomach,” “cancer” or “carcinoma” or “neoplasm” or “tumor,” “young adult” or “younger” or “youth.” These searches were limited to clinical articles published up to December 2019.
Inclusion and exclusion criteria
Studies met the following criteria were included: (I) researches compared gastric cancer in the younger group (≤40 years of age) and older group (>40 years of age); (II) analyses contained quantitative clinicopathological information; (III) researches involved at least one of the mentioned survival outcomes.
Studies were excluded from the analysis as follow: (I) publications were position papers, editorials, case reports, comments, or review articles; (II) literature duplication based on an author or center; (III) research data was inappropriate or cannot be extracted; (IV) studies lacked control group for meta-analysis.
Data extraction
Two independent reviewers extracted predesigned data from the included studies. The extracted information was as follows: Basic characteristics of the study, including authors, country, patient inclusion criteria, sample size, design as well as quality assessment; Clinicopathological characteristics of patients, including gender, tumor location, differentiation, Lauren type, Borrmann classification, pTNM stage, and therapeutic regimens (involving chemotherapy, total/subtotal gastrectomy, curative resection, and lymphadenectomy); Survival outcomes, including metastasis, recurrence, and the short or long-term survival rates on different clinical tumor stage. The stage of gastric cancer was based on the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging system. Lymphadenectomy was divided into D1 to D4, depending on the primary tumor location and removal of each lymph node station (32). Gastrectomy was defined as patients received surgery with or without D2 lymphadenectomy, while curative gastrectomy was defined as resection with D2 lymphadenectomy and a negative margin. The disagreement was resolved through discussion among the reviewers.
Quality assessment
The quality of the included studies was evaluated using The Newcastle-Ottawa Quality Assessment Scale (NOS) (33). The NOS checklist consisted of three major categories (selection, comparability, and outcome) with a maximum of nine stars. Each included study achieving six or more number of stars was graded high quality. Any disagreement was discussed to reach a consensus.
Statistical analysis
We conducted the review and meta-analysis using Revman software, version 5.3 (Cochrane Collaboration, Oxford, United Kingdom). Categorical variables were analyzed by the odds ratio (OR), while the corresponding 95% confidence interval (CI) was recorded. The Z test was conducted to determine the OR, with P<0.05 considered statistical significance. Heterogeneity was investigated using the χ2 test and the I2 test. If significant heterogeneity existed, we employed the random effect model; otherwise, the fixed effects model was adopted (34,35). Sensitivity analyses were undertaken to investigate sources of substantial heterogeneity.
Results
Studies selection
Our initial search strategy generated a total of 8,686 relevant clinical studies. After a screening of titles and abstracts, 108 articles were scrutinized by a full-text review. Eighty-three studies were eventually excluded by following the exclusion criteria and inclusion criteria. In total, the eligible 25 clinical studies (4,5,8-30) involving 81,188 gastric cancer patients were entered into the review and meta-analysis, of which one was a prospective study (17), three were multicenter studies (16,19,21), and the rest were all retrospective studies. Figure 1 showed the flow chart of the search process. The NOS scores and essential characteristics of the eligible studies were shown in Table 1.
Table 1
| Authors | Country | Patient criteria | Document type | NOS | Group | No. | Age | Gender | Tumor location | pTNM stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Upper | Middle | Lower | I | II | III | IV | ||||||||||
| Song et al. (4) | China | GC underwent surgery (2007–2011) | Retrospective Study | 7 | YG ≤40 | 112 | – | 59 | 53 | 12 | 21 | 64 | 5 | 30 | 59 | 18 | ||
| OG ≥70 | 358 | – | 274 | 84 | 61 | 64 | 208 | 25 | 89 | 206 | 38 | |||||||
| Cormedi et al. (5) | Brazil | GC (2011–2013) | Retrospective Study | 8 | YG ≤40 | 71 | 37 | 34 | 37 | – | – | – | 4 | 5 | 23 | 36 | ||
| OG >40 | 223 | 63.74 | 135 | 88 | – | – | – | 35 | 29 | 52 | 89 | |||||||
| Tavares et al. (8) | Portugal | GC with surgery (2000–2005) | Retrospective Study | 7 | YG ≤40 | 23 | – | 12 | 11 | – | – | – | 6 | 6 | 4 | 6 | ||
| OG >40 | 360 | – | 207 | 153 | – | – | – | 76 | 43 | 105 | 97 | |||||||
| Guan et al. (9) | the United States | GC (1973–2014) | Retrospective Study | 8 | YG <35 | 1,369 | – | 728 | 641 | 338 | 133 | 275 | 51 | 59 | 119 | 385 | ||
| OG ≥65 | 46,521 | – | 28,104 | 18,417 | 11,839 | 3,617 | 12,243 | 3,838 | 3,407 | 3,604 | 4,358 | |||||||
| Isobe et al. (10) | Japan | GAC (1977–2006) | Retrospective Study | 8 | YG ≤40 | 169 | 34.5±4.8 | 79 | 90 | 34 | 70 | 40 | 68 | 30 | 23 | 48 | ||
| OG >40 | 3,649 | 64.5±10.0 | 2,518 | 1,131 | 790 | 1,047 | 1,341 | 1,765 | 471 | 628 | 782 | |||||||
| Kim et al. (11) | Korea | GC (1986–2000) | Retrospective Study | 8 | YG ≤35 | 137 | 30.6±5.1 | 63 | 74 | 23 | 50 | 56 | 41 | 21 | 36 | 39 | ||
| OG >70 | 194 | 73.3±3.1 | 131 | 63 | 16 | 41 | 130 | 60 | 41 | 55 | 38 | |||||||
| Kunisaki et al. (12) | Japan | GC underwent curative surgery (1985–1999) | Retrospective Study | 8 | YG ≤40 | 131 | 35.2±5.0 | 64 | 67 | 44 | 65 | 19 | 79 | 16 | 24 | 12 | ||
| OG ≥55 | 918 | 60.2±3.2 | 658 | 260 | 340 | 386 | 168 | 510 | 123 | 174 | 111 | |||||||
| Liu et al. (13) | China | GC underwent surgery; no chemotherapy; no metastasis. (2008–2014) | Retrospective Study | 7 | YG ≤40 | 198 | – | 115 | 83 | – | – | – | – | – | – | – | ||
| OG ≥55 | 1,096 | – | 895 | 201 | – | – | – | – | – | – | – | |||||||
| Okamoto et al. (14) | Japan | GC underwent laparotomy (1960–1984) |
Retrospective Study | 6 | YG <30 | 34 | 24.9 | 10 | 24 | 3 | 13 | 7 | – | – | – | – | ||
| OG ≥75 | 132 | 77.9 | 97 | 35 | 12 | 37 | 66 | 39 | 7 | 31 | 55 | |||||||
| Takatsu et al. (15) | Japan | GC underwent surgical resection (2000–2010) | Retrospective Study | 8 | YG ≤40 | 136 | 36 [16–39] | 72 | 64 | 25 | 70 | 35 | 65 | 21 | 28 | 22 | ||
| OG ≥60 | 1,435 | 65 [60–69] | 1,024 | 411 | 385 | 581 | 416 | 786 | 206 | 253 | 190 | |||||||
| Tekesin et al. (16) | Turkey | GC (1990–2014) | Retrospective Cohort Study | 7 | YG ≤40 | 92 | 36 [22–40] | 53 | 39 | 17 | – | – | 5 | 4 | 17 | 52 | ||
| OG >40 | 774 | 60 [41–75] | 553 | 221 | 141 | – | –– | 25 | 46 | 195 | 372 | |||||||
| Wang et al. (17) | China | GC underwent gastrectomy (1998–2006) | Prospective Study | 7 | YG ≤40 | 21 | 34.9±1.1 | 9 | 12 | 1 | 7 | 13 | – | – | – | – | ||
| OG >55 | 36 | 67.1±0.8 | 22 | 14 | 4 | 7 | 25 | 11 | 9 | 15 | 1 | |||||||
| Hsieh et al. (18) | Japan | GAC underwent curative gastrectomy (1981–1992) | Retrospective Study | 7 | YG ≤40 | 115 | – | 46 | 69 | 14 | 27 | 68 | 23 | 22 | 56 | 14 | ||
| OG >60 | 1,009 | – | 626 | 373 | 194 | 160 | 626 | 293 | 160 | 467 | 89 | |||||||
| Ma et al. (19) | China | GC underwent curative surgery (2009–2011) | Retrospective Study | 7 | YG ≤40 | 125 | – | 76 | 49 | – | – | – | 30 | 24 | 71 | – | ||
| OG >40 | 1,752 | – | 1,341 | 411 | – | – | – | 403 | 400 | 946 | – | |||||||
| Mitsudomi et al. (20) | Japan | GC (1970–1984) | Retrospective Study | 7 | YG <40 | 128 | – | 66 | 62 | 13 | 58 | 31 | – | – | – | – | ||
| OG ≥50 | 1,275 | – | 863 | 412 | 131 | 379 | 550 | – | – | – | – | |||||||
| Kulig et al. (21) | Poland | GC (1977–1998) | Retrospective Study | 6 | YG ≤40 | 214 | 35.0 | 119 | 95 | 24 | 56 | 56 | 24 | 14 | 25 | 63 | ||
| OG >40 | 3,217 | 61.0 | 2,277 | 940 | 387 | 733 | 677 | 315 | 251 | 380 | 770 | |||||||
| Bani-Hani et al. (22) | Jordan | GAC (1991–2001) | Retrospective Study | 7 | YG ≤40 | 17 | 36.3±0.9 | 7 | 10 | 5 | 3 | 3 | 4 | 2 | 4 | 7 | ||
| OG >40 | 159 | 63.8±0.7 | 104 | 55 | 26 | 39 | 83 | 11 | 39 | 55 | 46 | |||||||
| Kim et al. (23) | Korea | GC underwent surgery (1993–2000) | Retrospective Study | 7 | YG ≤40 | 175 | 34.58±4.26 | 100 | 75 | 19 | 67 | 83 | 79 | 20 | 49 | 37 | ||
| OG >40 | 1,124 | 59.25±9.17 | 765 | 359 | 120 | 364 | 624 | 439 | 145 | 304 | 236 | |||||||
| Lai et al. (24) | Korea | GC underwent curative surgery (1987–2004) | Retrospective Study | 8 | YG ≤40 | 883 | 35 | 476 | 407 | 125 | – | – | 444 | 135 | 213 | 91 | ||
| OG >40 | 6,071 | 58.7 | 4,195 | 1,876 | 720 | – | – | 2,850 | 1,057 | 1,567 | 597 | |||||||
| Maehara et al. (25) | Japan | GC underwent surgery (1965–1991) | Retrospective Study | 6 | YG <40 | 174 | 38.8±4.9 | 89 | 85 | 31 | 58 | 63 | – | – | – | – | ||
| OG >70 | 356 | 74.8±3.9 | 247 | 109 | 90 | 86 | 152 | – | – | – | – | |||||||
| Silva et al. (26) | Brazil | GAC (1988–2005) | Retrospective Study | 7 | YG ≤40 | 62 | – | 38 | 24 | 9 | – | 50 | 21 | – | 35 | – | ||
| OG >40 | 453 | – | 288 | 165 | 68 | – | 385 | 127 | – | 280 | – | |||||||
| Zhou et al. (27) | China | GC resections (2004–2014) | Retrospective Study | 7 | YG ≤40 | 152 | 33.7±5.54 | 53 | 99 | 8 | 57 | 75 | 39 | 32 | 66 | 15 | ||
| OG >40 | 250 | 62.9±10.4 | 178 | 72 | 75 | 53 | 115 | 141 | 35 | 52 | 22 | |||||||
| Adachi et al. (28) | Japan | GC underwent surgery (1981–1990) | Retrospective Study | 7 | YG <40 | 36 | – | 20 | 16 | – | – | 6 | 16 | 5 | 8 | 7 | ||
| OG >60 | 68 | – | 43 | 25 | – | – | 27 | 25 | 13 | 16 | 14 | |||||||
| Bautista et al. (29) | the United States | Non-cardia GAC (2000–2010) | Retrospective Cohort Study | 8 | YG <40 | 46 | 34.1±4.1 | 24 | 22 | 1 | 10 | 14 | – | – | – | – | ||
| OG ≥50 | 1,208 | 71.5±3.8 | 714 | 494 | 65 | 379 | 426 | – | – | – | – | |||||||
| Wang et al. (30) | China | GC underwent curative gastrectomy (2005–2010) | Retrospective Study | 8 | YG ≤40 | 342 | 34.1±5.2 | 198 | 144 | 53 | 79 | 177 | 82 | 97 | 137 | 26 | ||
| OG >40 | 3,588 | 61.4±10.1 | 2,448 | 1,140 | 841 | 741 | 1,783 | 876 | 927 | 1,522 | 263 | |||||||
No., number of patients; pTNM, pathological (p), primary tumor (T), lymph nodes (N) and distant metastases (M); GC, gastric cancer; GAC, gastric adenocarcinoma.
Clinicopathological characteristics
The clinicopathologic characteristics of the gastric cancer patients were presented in Tables 2 and S1. Compared with the older group, younger patients with gastric cancer were more often female from pooled 25 studies (OR =2.09, 95% CI: 1.81–2.41, P<0.001, I2=76%) (Figure S1). Younger patients were more likely to be a diffuse type (OR =4.29, 95% CI: 3.15–5.85, P<0.001, I2=82%), pTNM stage IV (OR =1.21, 95% CI: 1.08–1.35, P<0.001, I2=0), poorly differentiation (OR =3.59, 95% CI: 2.89–4.47, P<0.001, I2=82%), and a signet ring cell carcinoma (OR =4.81, 95% CI: 4.33–5.33, P<0.001, I2=0) (Figure S2).
Table 2
| Subgroup | Included studies | Included patients | I2 (%) | Effect model | OR/WMD | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Female | 25 | 81,188 | 76 | Random | 2.09 | 1.81–2.41 | <0.001 |
| Diffuse type | 10 | 56,335 | 82 | Random | 4.29 | 3.15–5.85 | <0.001 |
| pTNM stage IV | 16 | 26,202 | 0 | Fixed | 1.21 | 1.08–1.35 | <0.001 |
| Poorly differentiation | 19 | 75,349 | 82 | Random | 3.59 | 2.89–4.47 | <0.001 |
| SRCC | 5 | 52,262 | 0 | Fixed | 4.81 | 4.33– 5.33 | <0.001 |
| Therapeutic regimen | |||||||
| Subtotal gastrectomy | 9 | 14,427 | 39 | Fixed | 0.88 | 0.79–0.99 | 0.03 |
| Curative gastrectomy | 14 | 18,159 | 10 | Fixed | 0.93 | 0.82–1.06 | 0.30 |
| D1 lymphadenectomy | 4 | 7,387 | 25 | Fixed | 0.59 | 0.48–0.73 | <0.001 |
| ≥ D2 lymphadenectomy | 4 | 7,387 | 27 | Fixed | 1.77 | 1.44–2.18 | <0.001 |
| Chemotherapy | 6 | 8,750 | 43 | Fixed | 1.79 | 1.49–2.16 | <0.001 |
| Postoperative complications | 5 | 6,309 | 73 | Random | 0.44 | 0.24–0.79 | 0.006 |
| Recurrence/metastasis | |||||||
| Peritoneal recurrence | 4 | 1,965 | 11 | Fixed | 1.93 | 1.31–2.84 | 0.001 |
| Lymph node metastasis | 8 | 3,901 | 0 | Fixed | 0.83 | 0.69–0.98 | 0.03 |
| Hepatic metastasis | 9 | 11,126 | 0 | Fixed | 0.68 | 0.47–0.98 | 0.04 |
| Peritoneal metastasis | 9 | 11,695 | 63 | Random | 1.63 | 1.16–2.27 | 0.004 |
| 5-year OS | 9 | 59,647 | 60 | Random | 1.01 | 0.79–1.30 | 0.92 |
| 5-year OS underwent surgery | 18 | 26,770 | 56 | Random | 1.35 | 1.16–1.57 | <0.001 |
| Stage I-OS | 8 | 6,536 | 11 | Fixed | 2.38 | 1.56–3.61 | <0.001 |
| Stage II-OS | 8 | 3,347 | 46 | Fixed | 1.28 | 0.98–1.66 | 0.07 |
| Stage III-OS | 7 | 5,702 | 27 | Fixed | 1.36 | 1.14–1.63 | <0.001 |
| Stage IV-OS | 7 | 1,483 | 0 | Fixed | 1.93 | 1.30–2.85 | 0.001 |
| 5-year OS underwent curative surgery | 12 | 19,012 | 60 | Random | 1.39 | 1.12–1.72 | 0.002 |
| Stage I-OS | 4 | 5,261 | 51 | Random | 1.73 | 0.86–3.49 | 0.13 |
| Stage II-OS | 4 | 2,771 | 51 | Random | 0.95 | 0.60–1.51 | 0.83 |
| Stage III-OS | 4 | 4,639 | 0 | Fixed | 1.29 | 1.05–1.58 | 0.01 |
| Stage IV-OS | 3 | 1,016 | 0 | Fixed | 1.86 | 1.20–2.89 | 0.006 |
pTNM, pathological (p), primary tumor (T), lymph nodes (N) and distant metastases (M); SRCC, signet ring cell carcinoma; OS, overall survival.
Concerning to therapeutic regimen, six studies showed that younger group had a higher chemotherapy rate when compared to older group (OR =1.79, 95% CI: 1.49–2.16, P<0.001, I2=43%). In addition, the proportions of younger patients underwent subtotal gastrectomy or D1 resection were significantly lower than those of the older (OR =0.88, 95% CI: 0.79–0.99, P=0.03, I2=39%; OR =0.59, 95% CI: 0.48–0.73, P<0.001, I2=25%, respectively). However, there were no statistical differences in curative resection rate between the two groups (OR =0.93; 95% CI: 0.82–1.06, P=0.30, I2=10%) (Figure S3).
Postoperative complications
A total of 6,309 patients from five studies were enrolled in postoperative complications. The result revealed that the proportion of complications in younger patients was significantly lower compared to the older (OR =0.44, 95% CI: 0.24–0.79, P=0.006), and the heterogeneity between the younger and older group was significant (I2=73%) (Figure S4).
Survival outcomes
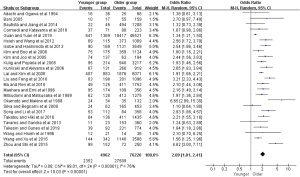
Figure 2 presented the meta-analysis of the 5-year overall survival (OS) with total patients, gastrectomy group, and only curative gastrectomy group, respectively. There was no significant difference for total patients based on the nine included studies (OR =1.01, 95% CI: 0.79–1.30, P=0.92, I2=60%). However, the pooled 18 and 12 studies respectively showed that younger adults in gastrectomy group and only curative gastrectomy group were associated with better survival relative to that of the older (OR =1.35, 95% CI: 1.16–1.57, P<0.001, I2=56%; OR =1.39, 95% CI: 1.12–1.72, P=0.002, I2=60%).
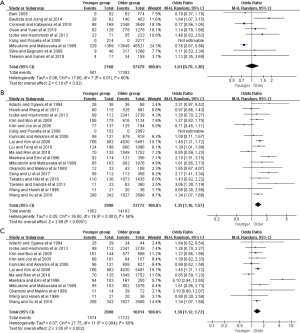
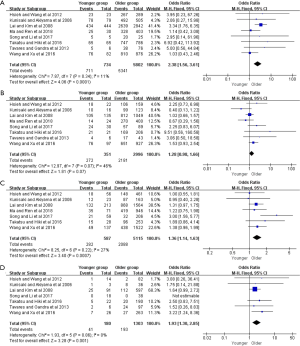
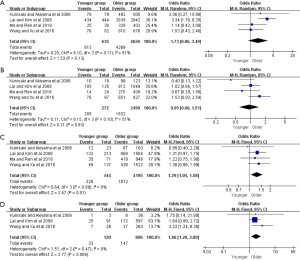
Moreover, further survival analyses between younger and older patients were done under the different pTNM tumor stage. Four of the studies provided survival rates for gastrectomy group, and the meta-analysis showed that younger patients at pTNM stage I, stage III, and stage IV were associated with better 5-year OS than older (OR =2.38, 95% CI: 1.56–3.61, P<0.001, I2=11%; OR =1.36, 95% CI: 1.14–1.63, P<0.001, I2=27%; OR =1.93, 95% CI: 1.30–2.85, P=0.001, I2=0%, respectively) (Figure 3). For the only curative gastrectomy group, three of the included studies revealed that younger patients at pTNM stage III and stage IV also had improved survival (OR =1.29, 95% CI: 1.05–1.58, P=0.01, I2=0%; OR =1.86, 95% CI: 1.20–2.89, P=0.006, I2=0%, respectively), but there was no statistical difference in gastric cancer at stage I (OR =1.73, 95% CI: 0.86–3.49, P=0.13, I2=51%) (Figure 4). The short-term (including the 1-, 2-, 3-year) survival rates were presented in Table S2.
Concerning to the metastasis status of gastric cancer, nine of the 25 studies showed that younger group was predominant in peritoneal metastasis (OR =1.63, 95% CI: 1.16–2.27, P=0.004, I2=63%). Some included studies reported the lymph node metastasis and hepatic metastasis of gastric cancer, and our result showed that both lymph node metastasis and hepatic metastasis ratio was lower in younger group compared with those of the older (OR =0.83, 95% CI: 0.69–0.98, P=0.03, I2=0%; OR =0.68, 95% CI: 0.47–0.98, P=0.04, I2=0%). In addition, 4 related studies indicated that the incidence of peritoneal recurrence was significantly higher in younger group (OR =1.93, 95% CI: 1.31–2.84, P=0.001, I2=11%) (Figure S5 and Table S3).
Discussion
The review and meta-analysis involved 24 retrospective comparative trails and one prospective study with 81,188 patients with gastric cancer. Our findings demonstrated that the younger group after gastrectomy or only curative gastrectomy was correlated with a better OS, but there was no significant difference for total patients between the two groups. To our best knowledge, this analysis was the most extensive evaluation to compare the clinicopathological feature and prognosis between the younger and older group.
Several findings regarding the clinicopathological characteristics in the meta-analysis were in agreement with previous researches, including a higher proportion of female, poorly differentiation, signet ring cell carcinoma, diffuse histology, and pTNM tumor stage IV in younger adults (8-21). Our survey revealed that younger patients had a higher proportion of females, while male predominance was mostly seen in the older group. Although the reasons for female predominance in younger patients were not clear, some potential explanations had been identified. Several studies considered hormonal factors, such as estrogens and higher percentages of estrogen receptor-positive cells might be associated with the predominance of younger females (36,37). Compared to older patients, younger patients with gastric cancer had been believed to be related to genetic changes rather than environmental factors (38). Thereby more frequent exposure to environmental carcinogens, such as cigarettes, might lead to the dominance among older male patients (39). Concerning to histological type, our analysis revealed that poorly differentiation, diffuse-type, and signet ring cell carcinoma were predominant in the younger group. In comparison, more patients in the older group were diagnosed as intestinal type and mucous adenocarcinoma. The primary reason may be germline mutations, specifically in the CDH1 gene, as reported in some researches (26,40,41). While the included studies rarely capture the duration of symptoms before initial diagnosis, other researches have reported delayed diagnosis, and hereditary factors may be closely correlated with advanced gastric cancer (42,43).
Surgery, especially curative resection, was an important approach for patients with gastric cancer (44). There were higher proportions of chemotherapy and ≥ D2 lymphadenectomy in the younger group compared with the older. However, the percentages of total gastrectomy and curative resection revealed no statistical differences between younger and older groups, while subtotal gastrectomy was frequently performed in older patients. These results may be due to the significant comorbidities and impairment of functional status in older patients (45-47). Moreover, a previous study demonstrated that the ratio of older patients who had other synchronous or previous malignancies at initial diagnosis was up to 21% based on Munich Cancer Registry data (48). In our review, postoperative complications were more prevalent in the older group, which also reflected a worse tolerance for surgery or chemotherapy. Several studies investigated that the incidence of postoperative complications was closely correlated with poor prognosis (49,50), thus providing a survival advantage for the younger group.
In this analysis, a tendency of peritoneal metastasis in the younger group may reflect the genetic susceptibility, such as CDH1 and RhoA, that could lead to more aggressive biological behaviors (40,51). Moreover, the infiltration of poorly differentiated gastric cancer was more pronounced in the vertical direction, thus conferring lymph node involvement and peritoneal dissemination. Metastasis was the leading cause of recurrence, and it had been thought that peritoneal metastasis was the most common form of repetition in gastric carcinoma (15). Our finding indicated a higher incidence of peritoneal recurrence in younger patients, which was similar to the other conclusion (12).
Younger gastric cancer patients as a group revealed similar long-term OS compared to older, and this finding was consistent with previous studies (5,10,11,20). In the subgroups of gastrectomy and only curative gastrectomy, both the short-term (including the 1-, 2-, 3-year) and long-term (including the 5-year) OS for older group was more miserable than those of the younger group, possibly due to a more significant percentage of comorbidities and complications. When the 5-year OS under different pTNM stages was evaluated, the results differed substantially between the younger and older group. A trend towards better long-term survival in the younger group may reflect a higher tolerance for the patients given a younger age and fewer comorbidities. Moreover, the shorter life expectancy of the older group compared to the younger may also be responsible.
There were several limitations in the analysis because of the characteristics of the included studies identified. Firstly, only one of the trials we identified was a prospective study. Secondly, most of the included studies were from Eastern Asia, which might not have a great representative and guiding value across the globe, especially in Western countries. Thereby, more related researches were expected to evaluate in gastric cancer patients at a younger age. Thirdly, there were inevitable heterogeneities, such as female ratio, diffuse type, as well as several survival variables in the analysis. The contribution of each included study to the pooled estimate was evaluated in the sensitivity analyses, and the result showed that sources of these heterogeneities were mainly from the selection bias. Furthermore, the lack of available patient data did not allow our analysis to assess disease-specific survival and disease-free survival. Despite these limitations, the study to our knowledge was the most extensive analysis evaluating the clinicopathological characteristics and survival outcomes in the younger and older patients, which may overcome the limitation of small sample size and single-institution targeted the field. Besides, all of the clinical studies involved in the meta-analysis had a high quality and met our inclusion criteria, thus might provide more valuable resources for the clinicians in patients' management and decision-making.
Conclusions
In conclusion, younger patients with gastric cancer were more often diagnosed as poorly differentiation and later pTNM tumor stage. However, younger cancer patients following gastrectomy had a better OS rate than patients in older group. Future large-scale analyses are expected to confirm our findings.
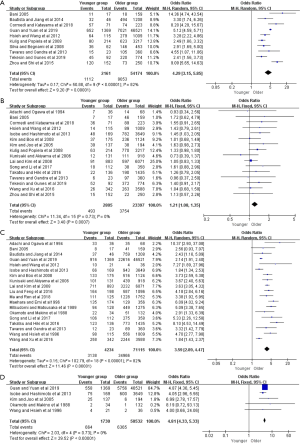
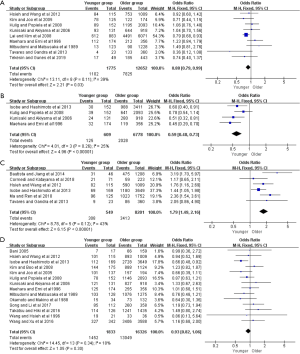
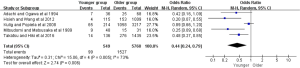
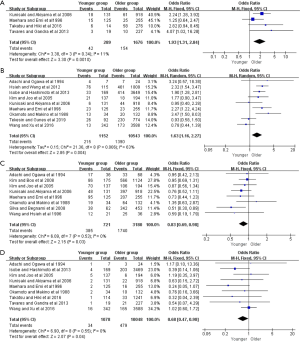
Table S1
| Authors | Group | No. | Tumor size ± SD (cm) | Pain | Bleeding | Cardiopulmonary disease | Differentiation | SRCC | Mucinous | Lauren type | Borrmann classification | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Well | Poor | Intestinal | Diffuse | Mixed | I | II | III | IV | ||||||||||
| Song et al. (4) | YG | 112 | ≤6 n=70; >6 n=42 | – | – | – | 6 | 106 | – | – | – | – | – | – | – | – | – | |
| OG | 358 | ≤6 n=239; >6 n=119 | – | – | – | 83 | 275 | – | – | – | – | – | – | – | – | – | ||
| Cormedi et al. (5) | YG | 71 | – | – | – | – | – | – | – | – | 3 | 57 | 3 | – | – | – | – | |
| OG | 223 | – | – | – | – | – | – | – | – | 78 | 74 | 14 | – | – | – | – | ||
| Tavares et al. (8) | YG | 23 | – | 12 | 3 | – | 4 | 12 | – | – | 8 | 15 | 0 | – | – | – | – | |
| OG | 360 | – | 160 | 100 | – | 56 | 89 | – | – | 255 | 105 | 0 | – | – | – | – | ||
| Guan et al. (9) | YG | 1,369 | 5.00±3.00 | – | – | – | 31 | 916 | 558 | 25 | 668 | 652 | – | – | – | – | – | |
| OG | 46,521 | 4.00±1.47 | – | – | – | 2493 | 22,616 | 5756 | 990 | 37,799 | 7,021 | – | – | – | – | – | ||
| Isobe et al. (10) | YG | 169 | – | – | – | – | – | 66 | 75 | 4 | – | – | – | – | – | – | – | |
| OG | 3,649 | – | – | – | – | – | 943 | 600 | 82 | – | – | – | – | – | – | – | ||
| Kim et al. (11) | YG | 137 | 5.07±3.23 | – | – | – | – | – | 25 | 4 | – | – | – | 5 | 13 | 93 | 26 | |
| OG | 194 | 5.16±3.45 | – | – | – | – | – | 6 | 10 | – | – | – | 10 | 43 | 128 | 13 | ||
| Kunisaki et al. (12) | YG | 131 | <5 n=76; ≥5 n=55 | – | – | – | 30 | 101 | – | – | – | – | – | – | – | – | – | |
| OG | 918 | <5 n=536; ≥5 n=382 | – | – | – | 479 | 439 | – | – | – | – | – | – | – | – | – | ||
| Liu et al. (13) | YG | 198 | – | – | – | 0 | 7 | 164 | – | – | – | – | – | – | – | – | – | |
| OG | 1,096 | – | – | – | 29 | 123 | 587 | – | – | – | – | – | – | – | – | – | ||
| Okamoto et al. (14) | YG | 34 | – | – | – | – | – | 22 | 2 | 0 | – | – | – | 0/20 | 0 | 12 | 4 | |
| OG | 132 | – | – | – | – | – | 51 | 1 | 5 | – | – | – | 3/85 | 25 | 34 | 14 | ||
| Takatsu et al. (15) | YG | 136 | – | – | – | – | 13 | 123 | – | – | – | – | – | – | – | – | – | |
| OG | 1,435 | – | – | – | – | 662 | 773 | – | – | – | – | – | – | – | – | – | ||
| Tekesin et al. (16) | YG | 92 | – | 22 | 6 | – | – | – | – | – | 39 | 45 | 7 | – | – | – | – | |
| OG | 774 | – | 191 | 52 | – | – | – | – | – | 526 | 220 | 21 | – | – | – | – | ||
| Wang et al. (17) | YG | 21 | <5 n=13; ≥5 n=8 | – | – | – | 4 | 10 | 4 | 1 | – | – | – | 1 | 6 | 12 | 2 | |
| OG | 36 | <5 n=23; ≥5 n=13 | – | – | – | 10 | 4 | 2 | 5 | – | – | – | 2 | 13 | 19 | 1 | ||
| Hsieh et al. (18) | YG | 115 | 4.80±3.50 | – | – | – | 17 | 98 | – | – | 17 | 64 | 13 | – | – | – | – | |
| OG | 1,009 | 4.50±3.00 | – | – | – | 453 | 556 | – | – | 491 | 279 | 103 | – | – | – | – | ||
| Ma et al. (19) | YG | 125 | – | – | – | – | 3 | 111 | – | – | – | – | – | – | – | – | – | |
| OG | 1,752 | – | – | – | – | 93 | 1,228 | – | – | – | – | – | – | – | – | – | ||
| Mitsudomi et al. (20) | YG | 128 | – | 48 | 6 | 3 | 5 | 94 | – | – | – | – | – | 2 | 11 | 28 | 20 | |
| OG | 1,275 | – | 20 | 3 | 14 | 600 | 449 | – | – | – | – | – | 20 | 175 | 347 | 106 | ||
| Kulig et al. (21) | YG | 214 | – | 90 | 12 | 2 | – | – | – | – | 42 | 80 | 18 | – | – | – | – | |
| OG | 3,217 | – | 1831 | 186 | 293 | – | – | – | – | 1,106 | 623 | 207 | – | – | – | – | ||
| Bani-Hani et al. (22) | YG | 17 | – | 12 | 2 | – | – | 8 | – | – | 6 | 11 | – | – | – | – | – | |
| OG | 159 | – | 109 | 23 | – | – | 41 | – | – | 121 | 18 | – | – | – | – | – | ||
| Kim et al. (23) | YG | 175 | – | – | – | – | 42 | 133 | – | – | – | – | – | – | – | – | – | |
| OG | 1,124 | – | – | – | – | 608 | 516 | – | – | – | – | – | – | – | – | – | ||
| Lai et al. (24) | YG | 883 | ≤4 n=586; >4 n=288 | – | – | – | 135 | 711 | – | – | – | – | – | 10 | 114 | 297 | 75 | |
| OG | 6,071 | ≤4 n=354; >4 n=2,488 | – | – | – | 2,661 | 3,232 | – | – | – | – | – | 665 | 812 | 2,039 | 405 | ||
| Maehara et al. (25) | YG | 174 | 7.10±4.20 | – | – | – | 39 | 135 | – | – | – | – | – | – | – | – | – | |
| OG | 356 | 6.30±3.80 | – | – | – | 225 | 129 | – | – | – | – | – | – | – | – | – | ||
| Silva et al. (26) | YG | 62 | ≤5 n=31; >5 n=27 | – | – | – | – | – | – | – | 15 | 36 | 11 | – | – | – | – | |
| OG | 453 | ≤5 n=179; >5 n=259 | – | – | – | – | – | – | – | 230 | 146 | 77 | – | – | – | – | ||
| Zhou et al. (27) | YG | 152 | – | 73 | 19 | – | – | – | – | – | 14 | 120 | 18 | – | – | – | – | |
| OG | 250 | – | 98 | 11 | – | – | – | – | – | 156 | 73 | 21 | – | – | – | – | ||
| Adachi et al. (28) | YG | 36 | 6 | 23 | – | 0 | – | 33 | – | – | – | – | – | – | – | – | – | |
| OG | 68 | 6.05 | 16 | – | 21 | – | 35 | – | – | – | – | – | – | – | – | – | ||
| Bautista et al. (29) | YG | 46 | – | – | – | 3 | 0 | 37 | – | – | 14 | 32 | – | – | – | – | – | |
| OG | 1,208 | – | – | – | 564 | 40 | 759 | – | – | 754 | 494 | – | – | – | – | – | ||
| Wang et al. (30) | YG | 342 | – | – | – | – | 16 | 258 | 86 | 16 | 64 | 166 | 112 | 18 | 114 | 156 | 54 | |
| OG | 3,588 | – | – | – | – | 172 | 2,244 | 534 | 233 | 790 | 2,049 | 1,027 | 272 | 1,252 | 1,756 | 308 | ||
No., number of patients; Pain, abdominal pain; SRCC, signet ring cell carcinoma; YG, younger group; OG, older group.
Table S2
| Subgroup | Included studies | Included patients | I2 (%) | Effect model | OR/WMD | 95% CI | P |
|---|---|---|---|---|---|---|---|
| OS | |||||||
| 1-year OS | 8 | 59,132 | 81 | Random | 1.08 | 0.80–1.45 | 0.63 |
| 2-year OS | 8 | 59,132 | 78 | Random | 1.04 | 0.79–1.36 | 0.79 |
| 3-year OS | 8 | 59,132 | 74 | Random | 1.01 | 0.78–1.32 | 0.93 |
| 5-year OS | 9 | 59,647 | 60 | Random | 1.01 | 0.79–1.30 | 0.92 |
| OS underwent gastrectomy | |||||||
| 1-year OS | 15 | 18,442 | 0 | Fixed | 1.20 | 1.04–1.39 | 0.01 |
| 2-year OS | 15 | 18,442 | 56 | Random | 1.31 | 1.08–1.58 | 0.005 |
| 3-year OS | 15 | 18,442 | 1 | Fixed | 1.33 | 1.19–1.48 | <0.001 |
| 5-year OS | 18 | 26,770 | 56 | Random | 1.35 | 1.16–1.57 | <0.001 |
| Stage I-OS underwent gastrectomy1 | |||||||
| 1-year OS | 5 | 5,437 | 0 | Fixed | 5.18 | 1.03–26.03 | 0.05 |
| 2-year OS | 5 | 5,437 | 0 | Fixed | 2.29 | 1.11–4.71 | 0.02 |
| 3-year OS | 5 | 5,437 | 0 | Fixed | 3.32 | 1.72–6.40 | <0.001 |
| 5-year OS | 8 | 6,536 | 11 | Fixed | 2.38 | 1.56–3.61 | <0.001 |
| Stage II-OS underwent gastrectomy | |||||||
| 1-year OS | 5 | 2,735 | 0 | Fixed | 1.54 | 0.72–3.33 | 0.27 |
| 2-year OS | 5 | 2,735 | 0 | Fixed | 1.25 | 0.80–1.94 | 0.33 |
| 3-year OS | 5 | 2,735 | 45 | Fixed | 1.47 | 1.01–2.14 | 0.04 |
| 5-year OS | 8 | 3,347 | 46 | Fixed | 1.28 | 0.98–1.66 | 0.07 |
| Stage III-OS underwent gastrectomy | |||||||
| 1-year OS | 5 | 4,499 | 61 | Random | 1.41 | 0.81–2.45 | 0.22 |
| 2-year OS | 5 | 4,499 | 55 | Random | 1.53 | 1.07–2.20 | 0.02 |
| 3-year OS | 5 | 4,499 | 60 | Random | 1.62 | 1.14–2.31 | 0.007 |
| 5-year OS | 7 | 5,702 | 27 | Fixed | 1.36 | 1.14–1.63 | <0.001 |
| Stage IV-OS underwent gastrectomy | |||||||
| 1-year OS | 5 | 1,341 | 74 | Random | 1.18 | 0.54–2.58 | 0.68 |
| 2-year OS | 5 | 1,341 | 83 | Random | 3.46 | 1.26–9.56 | 0.02 |
| 3-year OS | 5 | 1,341 | 41 | Fixed | 1.77 | 1.23–2.54 | 0.002 |
| 5-year OS | 7 | 1,483 | 0 | Fixed | 1.93 | 1.30–2.85 | 0.001 |
| OS underwent curative surgery | |||||||
| 1-year OS | 11 | 12,660 | 0 | Fixed | 1.35 | 1.05–1.72 | 0.02 |
| 2-year OS | 11 | 12,660 | 33 | Fixed | 1.22 | 1.03–1.45 | 0.02 |
| 3-year OS | 11 | 12,660 | 0 | Fixed | 1.36 | 1.17–1.58 | <0.001 |
| 5-year OS | 12 | 19,012 | 60 | Random | 1.39 | 1.12–1.72 | 0.002 |
| Stage I-OS underwent curative surgery | |||||||
| 5-year OS | 4 | 5,261 | 51 | Random | 1.73 | 0.86–3.49 | 0.13 |
| Stage II-OS underwent curative surgery | |||||||
| 5-year OS | 4 | 2,771 | 51 | Random | 1.07 | 0.80–1.43 | 0.67 |
| Stage III-OS underwent curative surgery | |||||||
| 5-year OS | 4 | 4,639 | 0 | Fixed | 1.29 | 1.05–1.58 | 0.01 |
| Stage IV-OS underwent curative surgery | |||||||
| 5-year OS | 3 | 1,016 | 0 | Fixed | 1.86 | 1.20–2.89 | 0.006 |
| OS underwent Non-curative surgery | |||||||
| 1-year OS | 3 | 268 | 70 | Random | 1.31 | 0.40–4.29 | 0.66 |
| 2-year OS | 3 | 268 | 38 | Fixed | 0.92 | 0.49–1.71 | 0.87 |
| 3-year OS | 3 | 268 | 0 | Fixed | 1.37 | 0.72–2.61 | 0.34 |
| 5-year OS | 3 | 268 | 0 | Fixed | 1.14 | 0.56–2.36 | 0.72 |
1stage, pTNM stage. OS, overall survival.
Table S3
| Authors | Group | No. | Type of gastrectomy | Resection margin | Lymphadenectomy | Chemotherapy | Complication | Peritoneal recurrence | Metastasis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtotal | Total | R0 | R1/R2 | D0 | D1 | ≥D2 | Lymph node | Vessel | Hepatic | Peritoneal | ||||||||
| Song et al. (4) | YG | 112 | – | – | 85 | 27 | – | – | – | – | – | – | – | – | – | – | ||
| OG | 358 | – | – | 260 | 98 | – | – | – | – | – | – | – | – | – | – | |||
| Cormedi et al. (5) | YG | 71 | – | – | – | – | – | – | – | 21 | – | – | – | – | – | – | ||
| OG | 223 | – | – | – | – | – | – | – | 59 | – | – | – | – | – | – | |||
| Tavares et al. (8) | YG | 23 | 4 | 19 | – | – | – | – | – | 9 | – | 3 | – | – | 1 | – | ||
| OG | 360 | 133 | 227 | – | – | – | – | – | 86 | – | 10 | – | – | 21 | – | |||
| Guan et al. (9) | YG | 1,349 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| OG | 46,521 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Isobe et al. (10) | YG | 169 | – | 52 | 112 | – | 3 | 30 | 119 | 69 | – | – | – | – | 4 | 33 | ||
| OG | 3,649 | – | 936 | 2,728 | – | 217 | 988 | 2,205 | 1,180 | – | – | – | – | 203 | 414 | |||
| Kim et al. (11) | YG | 137 | 78 | 47 | 101 | – | – | – | – | – | – | – | 70 | – | 5 | 21 | ||
| OG | 194 | 122 | 52 | 157 | – | – | – | – | – | – | – | 106 | – | 6 | 18 | |||
| Kunisaki et al. (12) | YG | 131 | 93 | 25 | 121 | – | – | 24 | 107 | – | – | 18 | 48 | 34 | 2 | 6 | ||
| OG | 918 | 644 | 274 | 827 | – | – | 280 | 638 | – | – | 61 | 397 | 332 | 22 | 44 | |||
| Liu et al. (13) | YG | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| OG | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Okamoto et al. (14) | YG | 34 | – | – | 15 | – | 10 | – | – | – | – | – | 19 | – | 2 | 13 | ||
| OG | 132 | – | – | 73 | – | 23 | – | – | – | – | – | 64 | – | 10 | 20 | |||
| Takatsu et al. (15) | YG | 126 | – | 32 | 114 | 22 | – | – | – | – | 14 | 6 | – | – | 1/114 | – | ||
| OG | 1,435 | – | 445 | 1,241 | 194 | – | – | – | – | 276 | 58 | – | – | 33/1,241 | – | |||
| Tekesin et al. (16) | YG | 92 | 17 | 32 | – | – | – | – | – | – | – | – | – | 29 | – | 26 | ||
| OG | 774 | 185 | 260 | – | – | – | – | – | – | – | – | – | 254 | – | 230 | |||
| Wang et al. (17) | YG | 21 | – | – | 19 | – | – | – | – | – | 4 | – | – | 20 | – | 76 | ||
| OG | 36 | – | – | 33 | – | – | – | – | – | 153 | – | – | 155 | – | 461 | |||
| Hsieh et al. (18) | YG | 115 | 84 | 31 | 101 | 14 | – | – | – | 82 | – | – | 12 | – | – | – | ||
| OG | 1,009 | 753 | 256 | 893 | 116 | – | – | – | 590 | – | – | 25 | – | – | – | |||
| Ma et al. (19) | YG | 125 | – | – | – | – | – | – | – | 96 | – | – | – | 43 | – | – | ||
| OG | 1,752 | – | – | – | – | – | – | – | 1,023 | – | – | – | 451 | – | – | |||
| Mitsudomi et al. (20) | YG | 128 | 13 | 29 | 103 | – | – | – | – | – | 9 | – | – | – | – | – | ||
| OG | 1,275 | 90 | 236 | 1,076 | – | – | – | – | – | 15 | – | – | – | – | – | |||
| Kulig et al. (21) | YG | 214 | 89 | 63 | 78 | 74 | – | 39 | 113 | – | 65 | – | – | – | – | – | ||
| OG | 3,217 | 1,195 | 898 | 1,146 | 947 | – | 641 | 1,452 | – | 1,058 | – | – | – | – | – | |||
| Bani-Hani et al. (22) | YG | 17 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | ||
| OG | 159 | – | – | 66 | – | – | – | – | – | – | – | – | – | – | – | |||
| Kim et al. (23) | YG | 175 | – | – | 144 | 31 | – | – | – | – | – | – | 86 | – | – | – | ||
| OG | 1,124 | – | – | 888 | 236 | – | – | – | – | – | – | 566 | – | – | – | |||
| Lai et al. (24) | YG | 883 | 612 | 262 | – | – | – | – | – | – | – | – | – | – | – | – | ||
| OG | 6,071 | 4,491 | 1,519 | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Maehara et al. (25) | YG | 174 | 112 | 62 | 125 | – | – | 32 | 141 | – | – | 15 | 95 | 15 | 2 | 23 | ||
| OG | 356 | 212 | 139 | 255 | – | – | 119 | 237 | – | – | 25 | 207 | 81 | 16 | 23 | |||
| Silva et al. (26) | YG | – | – | – | – | – | – | – | – | – | – | – | 38 | – | – | – | ||
| OG | – | – | – | – | – | – | – | – | – | – | – | 342 | – | – | – | |||
| Adachi et al. (28) | YG2 | 36 | – | – | – | – | – | – | – | – | 7 | – | 17 | – | 1/7 | 4/7 | ||
| OG3 | 68 | – | – | – | – | – | – | – | – | 25 | – | 33 | – | 3/24 | 7/24 | |||
| Bautista et al. (29) | YG | 46 | – | – | – | – | – | – | – | 31 | – | – | – | – | – | – | ||
| OG | 1,208 | – | – | – | – | – | – | – | 475 | – | – | – | – | – | – | |||
| Wang et al. (30) | YG | 342 | – | – | 327 | 15 | – | – | – | 267 | – | – | – | – | 16 | 13 | ||
| OG | 3,588 | – | – | 3,406 | 182 | – | – | – | 2,856 | – | – | – | – | 165 | 173 | |||
No., number of patients; YG, younger group; OG, older group; R, resection margin.
Acknowledgments
Funding: This study was funded in part by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2024
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2024). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Anderson WF, Camargo MC, Fraumeni JF Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723-8. [Crossref] [PubMed]
- Song S, Li C, Li S, et al. Clinicopathological features and prognoses in younger and older patients with gastric cancer. Onco Targets Ther 2017;10:4795-802. [Crossref] [PubMed]
- Cormedi MCV, Katayama MLH, Guindalini RSC, et al. Survival and prognosis of young adults with gastric cancer. Clinics (Sao Paulo) 2018;73:e651s. [Crossref] [PubMed]
- Nakamura R, Saikawa Y, Takahashi T, et al. Retrospective analysis of prognostic outcome of gastric cancer in young patients. Int J Clin Oncol 2011;16:328-34. [Crossref] [PubMed]
- Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg 2009;144:506-10. [Crossref] [PubMed]
- Tavares A, Gandra A, Viveiros F, et al. analysis of clinicopathologic characteristics and prognosis of gastric cancer in young and older patients. Pathol Oncol Res 2013;19:111-7. [Crossref] [PubMed]
- Guan WL, Yuan LP, Yan XL, et al. More attention should be paid to adult gastric cancer patients younger than 35 years old: extremely poor prognosis was found. J Cancer 2019;10:472-8. [Crossref] [PubMed]
- Isobe T, Hashimoto K, Kizaki J, et al. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep 2013;30:43-9. [Crossref] [PubMed]
- Kim DY, Joo JK, Ryu SY, et al. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol 2005;11:22-6. [Crossref] [PubMed]
- Kunisaki C, Akiyama H, Nomura M, et al. Clinicopathological features of gastric carcinoma in younger and middle-aged patients. a comparative study. J Gastrointest Surg 2006;10:1023-32. [Crossref] [PubMed]
- Liu S, Feng F, Xu G, et al. Clinicopathological features and prognosis of gastric cancer in young patients. BMC Cancer 2016;16:478. [Crossref] [PubMed]
- Okamoto T, Makino M, Kawasumi H, et al. Comparative study of gastric cancer in young and aged patients. Eur Surg Res 1988;20:149-55. [Crossref] [PubMed]
- Takatsu Y, Hiki N, Nunobe S, et al. Clinicopathological features of gastric cancer in young patients. Gastric Cancer 2016;19:472-8. [Crossref] [PubMed]
- Tekesin K, Emin Gunes M, Tural D, et al. Clinicopathological characteristics, prognosis and survival outcome of gastric cancer in young patients: a large cohort retrospective study. J BUON 2019;24:672-8. [PubMed]
- Wang JY, Hsieh JS, Huang CJ, et al. Clinicopathologic study of advanced gastric cancer without serosal invasion in young and old patients. J Surg Oncol 1996;63:36-40. [Crossref] [PubMed]
- Hsieh FJ, Wang YC, Hsu JT, et al. Clinicopathological features and prognostic factors of gastric cancer patients aged 40 years or younger. J Surg Oncol 2012;105:304-9. [Crossref] [PubMed]
- Ma X, Ren D, Kan J, et al. Clinicopathological Characteristics and Prognoses of Elderly Gastric Cancer Patients after R0 Resection. A Multicenter Study in China. J Environ Pathol Toxicol Oncol 2018;37:81-91. [Crossref] [PubMed]
- Mitsudomi T, Matsusaka T, Wakasugi K, et al. A clinicopathological study of gastric cancer with special reference to age of the patients. an analysis of 1,630 cases. World J Surg 1989;13:225-30; discussion 230-1. [Crossref] [PubMed]
- Kulig J, Popiela T, Kolodziejczyk P, et al. Clinicopathological profile and long-term outcome in young adults with gastric cancer: multicenter evaluation of 214 patients. Langenbecks Arch Surg 2008;393:37-43. [Crossref] [PubMed]
- Bani-Hani KE. Clinicopathological comparison between young and old age patients with gastric adenocarcinoma. Int J Gastrointest Cancer 2005;35:43-52. [Crossref] [PubMed]
- Kim JH, Boo YJ, Park JM, et al. incidence and long-term outcome of young patients with gastric carcinoma according to sex: does hormonal status affect prognosis? Arch Surg 2008;143:1062-7; discussion 1067. [Crossref] [PubMed]
- Lai JF, Kim S, Li C, et al. Clinicopathologic characteristics and prognosis for young gastric adenocarcinoma patients after curative resection. Ann Surg Oncol 2008;15:1464-9. [Crossref] [PubMed]
- Maehara Y, Emi Y, Tomisaki S, et al. Age-related characteristics of gastric carcinoma in young and elderly patients. Cancer 1996;77:1774-80. [Crossref] [PubMed]
- Silva EM, Begnami MD, Fregnani JH, et al. Cadherin-catenin adhesion system and mucin expression. a comparison between young and older patients with gastric carcinoma. Gastric Cancer 2008;11:149-59. [Crossref] [PubMed]
- Zhou F, Shi J, Fang C, et al. Gastric carcinomas in young (younger than 40 years) Chinese patients: clinicopathology, family history, and postresection survival. Medicine (Baltimore) 2016;95:e2873. [Crossref] [PubMed]
- Adachi Y, Ogawa Y, Sasaki Y, et al. A clinicopathologic study of gastric carcinoma with reference to age of patients. J Clin Gastroenterol 1994;18:287-90. [Crossref] [PubMed]
- Bautista MC, Jiang SF, Armstrong MA, et al. Impact of age on clinicopathological features and survival of patients with noncardia gastric adenocarcinoma. J Gastric Cancer 2014;14:238-45. [Crossref] [PubMed]
- Wang Z, Xu J, Shi Z, et al. Clinicopathologic characteristics and prognostic of gastric cancer in young patients. Scand J Gastroenterol 2016;51:1043-9. [Crossref] [PubMed]
- Kong X, Wang JL, Chen HM, et al. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol 2012;106:346-52. [Crossref] [PubMed]
- Jiang L, Yang KH, Chen Y, et al. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg 2014;101:595-604. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials. an update. Contemp Clin Trials 2007;28:105-14. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139-45. [Crossref] [PubMed]
- Camargo MC, Goto Y, Zabaleta J, et al. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21:20-38. [Crossref] [PubMed]
- Wang Z, Butler LM, Wu AH, et al. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int J Cancer 2016;138:2837-45. [Crossref] [PubMed]
- Liu Y, Kaneko S, Sobue T. Trends in reported incidences of gastric cancer by tumour location, from 1975 to 1989 in Japan. Int J Epidemiol 2004;33:808-15. [Crossref] [PubMed]
- Green J, Roddam A, Pirie K, et al. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort. Br J Cancer 2012;106:210-6. [Crossref] [PubMed]
- Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germline E-cadherin mutations. N Engl J Med 2001;344:1904-9. [Crossref] [PubMed]
- Suriano G, Yew S, Ferreira P, et al. Characterization of a recurrent germ line mutation of the E-cadherin gene: implications for genetic testing and clinical management. Clin Cancer Res 2005;11:5401-9. [Crossref] [PubMed]
- Koea JB, Karpeh MS, Brennan MF. Gastric cancer in young patients: demographic, clinicopathological, and prognostic factors in 92 patients. Ann Surg Oncol 2000;7:346-51. [Crossref] [PubMed]
- Chung HW, Noh SH, Lim JB. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol 2010;16:256-63. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Tsujitani S, Katano K, Oka A, et al. Limited operation for gastric cancer in the elderly. Br J Surg 1996;83:836-9. [Crossref] [PubMed]
- Korenaga D, Baba H, Kakeji Y, et al. Comparison of R1 and R2 gastrectomy for gastric cancer in patients over 80 years of age. J Surg Oncol 1991;48:136-41. [Crossref] [PubMed]
- Haga Y, Yagi Y, Ogawa M. Less-invasive surgery for gastric cancer prolongs survival in patients over 80 years of age. Surg Today 1999;29:842-8. [Crossref] [PubMed]
- Schlesinger-Raab A, Mihaljevic AL, Egert S, et al. Outcome of gastric cancer in the elderly: a population-based evaluation of the Munich Cancer Registry. Gastric Cancer 2016;19:713-22. [Crossref] [PubMed]
- Kubota T, Hiki N, Sano T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 2014;21:891-8. [Crossref] [PubMed]
- Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004;198:42-50. [Crossref] [PubMed]
- Carneiro F, Huntsman DG, Smyrk TC, et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol 2004;203:681-7. [Crossref] [PubMed]