
Clinicopathological characteristics and treatment outcome in obese patients with diffuse large B-cell lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive but highly curable disease. Current first-line treatment regimen employing rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) immunochemotherapy is remarkably effective, with more than 50% of the patients alive and disease-free at 5 years (1). However, despite the advances in therapy and the clearly defined standard approach for all patients, individuals with DLBCL have disparate outcomes based on varying demographic, clinical and biological factors. Risk stratification in patients with DLBCL is, therefore, of utmost importance. Traditionally, the international prognostic index (IPI) has been used as the standard for outcome prediction (2), but the model was developed before the advent of monoclonal antibody rituximab. Recently, a new prognostic model similarly incorporating several key clinical parameters, the National Comprehensive Cancer Network (NCCN) IPI, was proposed for DLBCL patients treated with R-CHOP (3). The enhanced NCCN-IPI clearly demonstrates superiority over the old IPI for risk stratification in patients with DLBCL treated in the rituximab era, and several subsequent reports (4,5), including ours (6), have confirmed its unparalleled predictive value.
Biologically, DLBCLs are a heterogeneous group of neoplasms that include subsets of tumors with different cells of origin (COO) (germinal center B-cell vs. non-germinal center B-cell) (7). Implications in this COO classification include distinct pathobiology, differential response to therapeutic agents, and contrasting survival outcome within the same DLBCL entity (8-10). More recently, double-hit lymphoma (DHL) was described as a new subgroup of DLBCL with both MYC and BCL2 gene rearrangements (11). When immunohistochemical (IHC) staining instead of the more sensitive fluorescent in situ hybridization (FISH) was used for assessment of BCL2 and MYC expression, the entity was referred as double-expressor large cell lymphoma (DEL) (11-14), although there existed quite some disparities between DHL and DEL (14). Clinically, both DHL and DEL have substantially dismal prognosis, as they progress rapidly in spite of aggressive therapies (11,14). Together with NCCN-IPI, the COO classification and the designation of DHL/DEL constitute the most important clinical and biological variables that powerfully predict the survival outcomes in patients with DLBCL.
Obesity has been associated with a significantly increased risk of development of non-Hodgkin’s lymphoma (15), especially DLBCL (16,17). Studies have explored the prognostic implication of obesity in DLBCL, but the conclusions are diverse and even contradicting each other (18-23). Importantly, one of the major flaws in these studies lies in that obesity has not been considered in the same context with those clinical and biological parameters, including NCCN-IPI, COO, and DHL/DEL. For this reason, we conducted this retrospective analysis to delineate the clinicopathological characteristics of obese patients with DLBCL. We also incorporated obesity into known prognostic predictors of DLBCL to investigate potential association between body mass index (BMI) at diagnosis and survival outcomes. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1362).
Methods
Study population
We retrospectively screened all patients with pathologically-confirmed DLBCL diagnosed and treated at our institute between 2004 and 2015. Only immunocompetent patients with adequate paraffin-embedded biopsy specimens for IHC staining were included. Information on a variety of clinical characteristics, including patient demographics, results of baseline hemograms and biochemical tests, disease stage, involvement of extranodal sites, treatment outcome, and survival status, was obtained and reviewed. Patients included in the study all signed an informed consent stating their permission for the use of the leftover tumor samples stored in the bio-bank of our institute. The study was approved by the Institutional Review Board of Chang-Gung Memorial Hospital (Taiwan) (IRB number: 201600159B0C601) and conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Risk groups, treatment, and outcome
Based on BMI, patients were categorized as obese (BMI ≥27 kg/m2) and non-obese (BMI <27 kg/m2). The definition of obesity followed the criteria published by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan (http://health99.hpa.gov.tw/OnlinkHealth/BMI.html; accessed Dec. 15th, 2017). In this defined criterion, the BMI cut-off points of 24.0–26.9, 27.0–29.9, 30.0–34.9 and ≥35 kg/m2 were designated as overweight, obese class I, obese class II, and obese class III, respectively. The NCCN-IPI, which substratified patients into low, low-intermediate (LI), high-intermediate (HI), and high risks, was used for risk stratification (3). Lymphomatous infiltration of the bone marrow, central nervous system, liver, gastrointestinal tract, or lung was considered as high-risk extranodal involvement, as defined in the NCCN-IPI model (3). Histological subtypes of DLBCL, categorized as either germinal center B-cell (GCB) like or non-GCB like, were defined as previously described (7). For baseline characteristics delineation, all eligible patients were included. For survival outcome comparison, only patients receiving R-CHOP-like immunochemotherapy were included for analysis. The response to treatment was assessed according to standard response criteria (24). Progression-free survival (PFS) was measured from the date of diagnosis to the date of first documented progression or last follow-up. Overall survival (OS) was measured from the date of diagnosis until death from any cause, with observation ending at the date of last contact for patients last known to be alive.
Tissue microarray and IHC staining
The diagnosis of DLBCL in all pathologic specimens was confirmed and reviewed by at least two hematopathologists with expertise at our institute. Tissue fixation and processing were performed using standard methods. Tissue microarrays that contained two representative 1-mm cores from each tumor were prepared using AutoTiss 1000 tissue microarrayer (EverBio Technology Inc., Taipei, Taiwan). IHC staining was performed as previously reported (25). Primary antibodies against BCL2 (Abcam, Cambridge, UK) and c-Myc (Abcam) were used at dilutions of 1/250 and 1/200, respectively. A high-sensitivity diaminobenzidine (DAB+) chromogenic substrate system, ab80436–EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC Kit (Abcam), was used for detection. All patient cases were stained and scored semiquantitatively in 10% increments by two observers (JL Liu and CE Huang) without knowledge of patient outcome or each other’s results of interpretation, and only lymphoma cells were scored. Previously established cutoff points used to define double-expressor lymphoma (≥50% BCL2-positive lymphoma cells and ≥40% MYC-positive lymphoma cells) (12,13) were used to stratify tumors as high BCL2 expression and high MYC expression, respectively. Representative slides of various staining results on IHC studies for BCL2 and MYC expression are shown in Figures S1,S2, respectively.
Statistical analysis
The Mann-Whitney test was used for continuous variables versus categorical variables. For comparison of the dichotomous variables, a Pearson chi-square or a Fisher’s exact test (for expected values of >5 or ≤5, respectively) was applied. The variables of PFS and OS were estimated by the Kaplan-Meier method. Differences between groups were calculated using the log-rank test for univariate analysis. Cox’s proportional hazards model was used for multivariate analysis to test independent prognostic factors on survival outcome. All calculations were performed using the Statistical Package of Social Sciences software (version 17.0; SPSS, Inc., Chicago, IL, USA). The level of statistical significance was set at 0.05 for all tests.
Results
Clinical characteristics of the patients
Overall, 93 patients with pathologically confirmed de novo DLBCL diagnosed and treated at our institute were included in the current study. Based on the availability of BMI, stainable tissues, and results on treatment response assessment, the case numbers included in individual analyses on related information were depicted in a flow chart (Figure 1). Eight patients who did not have initial body weights and heights recorded were excluded from the baseline characteristics comparison using BMI as the indicator. We stratified our patients into obese (BMI ≥27 kg/m2) and non-obese (BMI <27 kg/m2) groups. Fifteen (17.6%) out of the 85 patients were categorized as obese. Table 1 summarizes the comparison of the clinical and laboratory features between obese and non-obese patients. Obese patients were significantly younger (age in years, 55.2±12, vs. 65.1±16.9 in non-obese patients, P=0.034) and had lower platelet counts [(176±78)×109/L vs. (248±103)×109/L, P=0.013] than their counterparts. However, there were no significant differences between the two subgroups of patients with respect to patient’s gender, performance status, the results of biochemical tests [including serum albumin, β2-microglobulin, and lactate dehydrogenase (LDH) levels], and other hematological parameters (white cell counts and hemoglobin levels). In the assessment of disease phenotypes, the mean IPI scores, the mean NCCN-IPI scores, the percentages of patients with high-intermediate or high-risk diseases categorized by NCCN-IPI criteria, and the distribution of COO (GCB vs. Non-GCB), were not considerably different between obese and non-obese patients. The incidences of high-risk extranodal involvement, as defined in the NCCN-IPI model (3), were similar in both groups as well. Based on the results of IHC staining, more obese patients had high MYC-expressing DLBCL (20%, compared to 7.5% in non-obese patients). Nevertheless, the difference was not statistically significant (P=0.157). On the other hand, obese patients had a strikingly higher likelihood to harbor high BCL2-expressing lymphoma than their counterparts did (40% vs. 10.4%, P=0.005). And the probability of obese patients whose tumors exhibited either high BCL2 or high MYC expression (or both) was 46.7% (7/15), as compared to 16.9% (11/65, P=0.013) seen in non-obese patients.
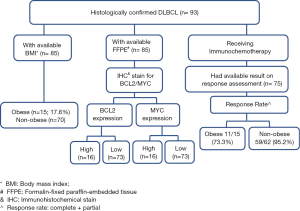
Table 1
| Variables | BMI, kg/m2 | P | |
|---|---|---|---|
| <27 (N=70) | ≥27 (N=15) | ||
| Age in years; mean ± SD | 65.1±16.9 | 55.2±12.0 | 0.034* |
| Males (%) | 41 (58.6) | 9 (60.0) | 0.919 |
| Performance status 0-1a; n (%) | 53 (75.7) | 13 (86.7) | 0.503 |
| LDHb level in U/L; mean ± SD | 245±253 | 416±500 | 0.232 |
| LDH level higher than normal (%) | 32 (47.8) | 10 (71.4) | 0.145 |
| β2-microglobulin, ng/mL; mean ± SD | 3,170±2,099 | 2,882±1,998 | 0.676 |
| Albumin, g/dL; mean ± SD | 3.4±0.8 | 3.6±0.7 | 0.285 |
| White cell count, ×109/L; mean ± SD | 7.7±3.0 | 6.3±2.1 | 0.088 |
| Hemoglobin, g/dL; mean ± SD | 11.7±1.9 | 12.8±2.8 | 0.150 |
| Platelet count, ×109/L; mean ± SD | 248±103 | 176±78 | 0.013* |
| IPIc score; mean ± SD | 2.22±1.39 | 2.13±1.51 | 0.823 |
| NCCN IPId score; mean ± SD | 3.51±1.88 | 3.47±1.81 | 0.939 |
| NCCN IPI high-intermediate & high risk; n (%) | 30 (44.8) | 8 (53.3) | 0.548 |
| Extranodal involvemente; n (%) | 35 (50.0) | 6 (40.0) | 0.482 |
| Cell of origin; n (%) | 0.122 | ||
| Germinal center B-cell like | 23 (37.7) | 2 (14.3) | |
| Non-germinal center B-cell | 38 (62.3) | 12 (85.7) | |
| MYC expression on IHCf stain; n (%) | 0.157 | ||
| Lowg | 62 (92.5) | 12 (80.0) | |
| Highg | 5 (7.5) | 3 (20.0) | |
| BCL2 expression on IHCf stain; n (%) | 0.005* | ||
| Lowh | 60 (89.6) | 9 (60.0) | |
| Highh | 7 (10.4) | 6 (40.0) | |
| BCL2 and MYC expression on IHCf stain; n (%) | 0.013* | ||
| BCL2low and MYClow | 54 (83.1) | 8 (53.3) | |
| BCL2high and/or MYChigh | 11 (16.9) | 7 (46.7) | |
a, ECOG performance status; b, LDH: lactate dehydrogenase; c, IPI: international prognostic index; d, NCCN-IPI: National Comprehensive Cancer Network International Prognostic Index; e, extranodal involvement of either BM, CNS, GI/liver, or lung; f, IHC stain: immunohistochemical stain; g, MYC expression: low: positive stain in <40% of cells; high: positive stain in ≥40% of cells; h, BCL2 expression: low: positive stain in <50% of cells; high: positive stain in ≥50% of cells. *, P value with significance.
In this 93-patient cohort, 89 had adequate paraffin-embedded biopsy specimens for IHC staining and were assessed for BCL2 and MYC expression. Overall, 16 patients (18.0%) had high BCL2-expressing DLBCL, and 8 patients (9.0%) had high MYC-expressing lymphoma. Based on the results of IHC staining, we further stratified our patients into different groups. The comparison on clinic-pathological features between different expressional levels of BCL2 and MYC were shown in Table S1 and S2, respectively. Patients with high BCL2 expression (≥50% BCL2-positive lymphoma cells) had higher BMI (27.0±6.8 vs. 23.3±3.7 kg/m2, P=0.073) and NCCN-IPI score (2.60±1.24 vs. 2.19±1.44, P=0.053) than those without, although the differences were only of borderline significance statistically. However, when using more clinically appropriate cutoff indicators including obesity (BMI ≥27 kg/m2) and NCCN-IPI stratified high-intermediate/high risks, more BCL2-positive patients were obese (46.2% vs. 13% P=0.005) or harbored a more aggressive tumor in the NCCN-IPI high-intermediate or high-risk categories (73.3% vs. 43.5% in non-obese patients, P=0.047, Table S1). On the other hand, other parameters were not significantly dissimilar between BCL2-high and BCL2-low patients.
We next checked the MYC staining result and its association with clinical parameters. However, as shown in Table S2, there were no apparent discrepancies among various characteristics in patients with DLBCL stratified by MYC expressional level. Because there were only 3 patients categorized as having double-expression (BCL2-high and MYC-high) DLBCL based on previously established criteria (12,13), we did not explore further on the features associated the unique subtype of DLBCL in our patient cohort.
Treatment and survival outcome analysis
In all, 82 patients with DLBCL received appropriate therapy and were therefore included in the outcome analysis. Among 77 patients who had response assessment after immunochemotherapy, the response rate (complete + partial) was considerably lower in obese patients (11/15, or 73.3%) than in non-obese patients (59/62, or 95.2%, P=0.024). The relapse rates among those who had a response, however, did not differ significantly between obese and non-obese patients (3/11 or 27.3%, vs. 11/59 or 18.6%, P=0.681).
Traditionally, NCCN-IPI, COO, BMI, and the expressional status of MYC and BCL2 have all been associated with the treatment outcome in patients with DLBCL (3,11,22,26). Therefore, we next evaluated these factors with respect to their impacts on PFS. As demonstrated in Figure 2, patients with diseases in the high-intermediate or high NCCN-IPI risk categorizes (P=0.029, Figure 2A), patients designated as obese based on their BMI (P=0.022, Figure 2B), patients with high BCL2 expression (P=0.000, Figure 2C), and patients with high MYC expression (P=0.001, Figure 2D) all had a far more dismal PFS outcome when compared to their respective counterparts on univariate analyses using Kaplan-Meier estimates. On taking BCL2 and MYC together into consideration, DLBCL patients whose tumors harbored overexpression of either protein fared significantly worse than those with BCL2/MYC low-expressing tumors (P=0.000, Figure 2E). On the other hand, the PFS difference between GCB-like and non-GCB-like DLBCLs was not statistically significant (P=0.251, Figure 2F). To test various parameters with possible prognostic value, we incorporated factors that showed potential values in PFS outcome prediction (including NCCN-IPI, BMI, and BCL2/MYC expression) into multivariate analysis using a Cox regression model to examine their potential interaction and effects on PFS. As demonstrated in Table 2, obesity (BMI > 27 kg/m2) was no longer relevant in predicting a PFS outcome (hazard ratio: 1.81, 95% CI: 0.67–4.91, P=0.242, Table 2), whereas BCL2/MYC expressional levels and NCCN-IPI risk categories remained prognostically important.
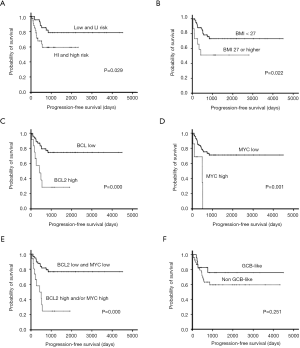
Table 2
| Variables | Progression-free survival, HR (95% CI) | P value |
|---|---|---|
| BCL2 and MYCa | 0.004* | |
| BCL2low and MYClow | 1.0 | |
| BCL2high and/or MYChigh | 4.12 (1.56–10.88) | |
| Body mass index, kg/m2 | 0.242 | |
| <27 | 1.0 | |
| ≥27 | 1.81 (0.67–4.91) | |
| NCCN-IPIb risk | 0.034* | |
| Low or low-intermediate | 1.0 | |
| High-intermediate or high | 2.83 (1.08–7.37) |
a, MYC expression: low: positive stain in <40% of cells; high: positive stain in ≥40% of cells; a, BCL2 expression: low: positive stain in <50% of cells; high: positive stain in ≥50% of cells; b, NCCN-IPI: National Comprehensive Cancer Network International Prognostic Index. *,P value with significance. HR, hazard ratio; CI, confidence interval.
We also evaluated the impacts of these variables on OS outcome. Univariate analyses similarly disclosed that patients with high-intermediate or high NCCN-IPI risk diseases (P=0.027, Figure 3A), high BCL2-expressing tumors (P=0.000, Figure 3B), and high MYC-expressing tumors (P=0.013, Figure 3C) all had an inferior OS. The same held true when BCL2 and MYC were grouped together as a unified parameter (P=0.000, Figure 3D). Non-obese patients had a better outcome, but the OS comparison between them and obese patients showed borderline significance only (P=0.064, Figure 3E). For the cell-or-origin comparison, there was no significant difference between GCB-like and non-GCB-like DLBCLs (P=0.117, Figure 3F). We then included NCCN-IPI, BMI, and BCL2/MYC expression status, three factors that were potentially prognostic significant on Kaplan-Meier OS estimates in the Cox regression model. On multivariate analysis, obesity was not an independent predictor for OS outcome (hazard ratio: 1.65, 95% CI: 0.58–4.73, P=0.352; Table 3), whereas BCL2/MYC expressional status and NCCN-IPI risk categories carried strong prognostic indications (Table 3).
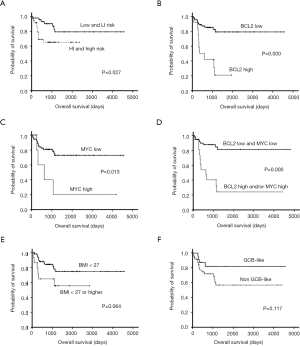
Table 3
| Variables | Overall survival, HR (95% CI) | P value |
|---|---|---|
| BCL2 and MYCa | 0.001* | |
| BCL2low and MYClow | 1.0 | |
| BCL2high and/or MYChigh | 5.63 (2.01–15.79) | |
| Body mass index, kg/m2 | 0.352 | |
| <27 | 1.0 | |
| ≥27 | 1.65 (0.58–4.73) | |
| NCCN-IPIb risk | 0.034* | |
| Low or low-intermediate | 1.0 | |
| High-intermediate or high | 3.11 (1.09–8.84) |
a, MYC expression: low: positive stain in <40% of cells; high: positive stain in ≥40% of cells; a, BCL2 expression: low: positive stain in <50% of cells; high: positive stain in ≥50% of cells; b, NCCN-IPI: National Comprehensive Cancer Network International Prognostic Index. *, P value with significance. HR, hazard ratio; CI, confidence interval.
With the relatively long enrollment period of our study cohort, we also assessed whether “period effects” had any impact on the treatment outcomes. We divided our patients into two subgroups based on the date of lymphoma diagnosis (before or after 2011). Further analyses revealed that the date of diagnosis did not affect key outcome parameters including response rate, PFS, and OS (data not shown).
Discussion
In the current study, we incorporated the NCCN-IPI, the COO, and the DEL proteins BCL2 and MYC to present important information on the clinical features and prognostic evaluation in obese patients with DLBCL treated in the rituximab era.
The most striking finding in our work is the strong association between obesity and the presence of either one of the DEL proteins BCL2 and MYC. Using the well-established cutoff values of ≥50% BCL2-positive lymphoma cells and ≥40% MYC-positive lymphoma cells on IHC stains to define positivity (12,13), we found that obese patients were 3 times more likely to harbor either BCL2- or MYC-positive tumors than non-obese patients (46.7% vs. 16.9%, P=0.013, Table 1). Although obesity has been a documented risk factor for the development of DLBCL (16,17), there have been no direct link between obesity and BCL2-positive or MYC-positive lymphomas. The mechanism behind such a correlation is not immediately clear. However, there is abundant data demonstrating obesity-related MYC- and BCL2-upregulation in solid tumors. In an in vivo mouse model, subcutaneously injected gastric cancer cells in diet-induced obese mice exhibited up-regulation of MYC protein (27). By co-culturing primary breast cancer cells with human adipocytes, Picon-Ruiz et al. showed that there were up-regulated MYC expression, increased tumor-initiating cell population and enhanced metastatic progression in these cancer cells (28). Indirect evidence included that leptin, an adipocyte-derived hormone which level could be increased in obese individual, induced the expression of BCL2 (29) and MYC (30) when it was used to treat breast cancer cells. Furthermore, genome-wide association studies have identified an interaction effect between BMI and BCL2 genotype (31). Therefore, it is plausible that obesity also plays a non-redundant role in the development of BCL2- or MYC-overexpressing DLBCL. Future exploratory bench work and confirmatory studies from larger DLBCL patient population are warranted to clarify such a correlation.
There have been some controversies regarding the prognostic impact of obesity on the clinical outcome of patients with DLBCL. In one of the earliest work investigating this issue, Geyer et al. found obesity was associated with decreased survival in DLBCL, but the study enrolled patients treated in the pre-rituximab era (18). Retrospective analyses by Carson et al. (20), Weiss et al. (21), and Ganti et al. (23), which included hundreds to thousands of DLBCL patients treated with immunochemotherapy, all described that patients with high BMI had a better clinical outcome as shown by either improved OS or decreased treatment-related mortality. However, two of the studies were flawed by not incorporating key DLBCL prognostic factors such as extra-nodal involvement and performance status into their multivariate analysis for survival outcome (20,23). Weiss et al. (21) did take IPI into consideration to clarify its interaction with obesity on the outcome prediction. Nevertheless, NCCN-IPI has been confirmed as a better prognostic model with well-recognized superiority over the old IPI for risk assessment in DLBCL patients treated with rituximab-based therapy (3,6). More importantly, none of these studies included biological factors inherent to lymphomas such as DHL/DEL in the Cox-regression proportional hazard model, so the results could be somewhat biased. In our work, obesity seemed to portend a dismal outcome in DLBCL, which included inferior response to treatment and shorter PFS as well as OS. But by combining with well-defined prognostic indicators such as NCCN-IPI and BCL2/MYC expression in multivariate analysis, the obesity factor became irrelevant in survival outcome prediction, whereas NCCN-IPI higher risks and BCL2/MYC over-expression still retained their adverse prognostic values. It is likely that two confounding factors associated with obesity, namely younger age and higher rate of BCL2/MYC overexpression, intertwine with each other and obesity, and this leads to the ambiguity of outcome-altering potential of obesity in DLBCL. Our result of a redundant role of obesity in clinical outcome prediction among patients with DLBCL best echoes the findings on a post-hoc analysis of the E4494 study (22). Although NCCN-IPI and biological factors were not included in their analysis, the investigators from that study did meticulously adjust obesity for IPI, gender, and rituximab. Considering the prospective nature of the E4494 trial, the conclusion of BMI being a non-factor in that study might be less biased and more apprehensible.
The definition of obesity in our study differs from that in Western countries. In the original proposal by a World Health Organization (WHO) expert committee meeting held in 1993, the proposed cut-off points of BMI for defining obesity was ≥30 kg/m2 (32). However, as scientific evidence suggests that Asian populations have different associations between BMI and health risks than do European populations, it has been widely accepted that the cut-off points for overweight and obesity in Asian people should be substantially lower (32). Since available data do not necessarily indicate a clear BMI cut-off point for all Asians for obesity, we follow the guideline published by the Ministry of Health and Welfare of Taiwan and use a BMI value of 27 kg/m2 or higher to define obese patients.
It is not immediately clear why our obese patients with DLBCL presented with lower platelet counts. However, considering that platelet count per se had not been linked to the prognosis prediction in DLBCL, we did not elaborate further on this aspect. Whether obesity is associated with the alteration of platelet counts probably requires large cohort study in general population to identify any potential relationship between these two factors.
Despite systemically providing invaluable information regarding the clinical characteristics as well as the prognosis of obese patients with DLBCL, our study was nevertheless limited in several aspects, notably its retrospective nature and the inclusion of an insufficient number of cases. Importantly, only three cases in our patient cohort were considered DE lymphoma based on previously published criteria (12,13), hence it’s impossible for us to determine whether DEL occurred more frequently in obese patients. Moreover, there seemed to be a trend of better PFS and OS in our patients with GCB-like DLBCL (Figures 2F,3F, respectively), but the number of patients with GCB vs. non-GCB stratification was too small to show a significant difference. That’s the major reason we did not include COO into our multivariate analysis on survival outcome. Further, various host/environmental factors and different ethnic background might lead to disparate phenotypes in obese patients with DLBCL. To solve those controversies, future prospective cohort studies involving many uniformly treated DLBCL patients are needed to better define this subgroup of patients. Work on dissecting the molecular mechanism of obesity-associated lymphomagenesis is also warranted to improve the treatment outcome in these patients.
Conclusions
In conclusion, our study has demonstrated that obese DLBCL patients exhibit unique clinical phenotypes shown by higher likelihood of harboring BCL2- or MYC-overexpressing tumors and an inferior response rate to immunochemotherapy. However, obesity loses its prognostic predictive value regarding PFS and OS when considered in the same context with NCCN-IPI and BCL2/MYC expression, as the latter two remain the most important factors in the prognostication of clinical outcomes in patients with DLBCL.
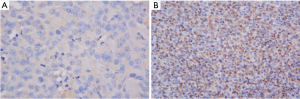
Table S1
| Variables | BCL2 | P | |
|---|---|---|---|
| <50 (N=73) | ≥50 (N=16) | ||
| Age in years; mean ± SD | 64.0±17.2 | 66.2±14.1 | 0.640 |
| Males (%) | 41 (56.2) | 10 (62.5) | 0.643 |
| Performance status 0-1a; n (%) | 54 (76.1) | 11 (68.8) | 0.538 |
| BMIb, kg/m2; mean ± SD | 23.3±3.7 | 27.0±6.8 | 0.073 |
| BMI ≥27 kg/m2; n (%) | 9 (13.0) | 6 (46.2) | 0.005* |
| LDHc level in U/L; mean ± SD | 215±180 | 541±587 | 0.060 |
| LDHc level higher than normal (%) | 32 (47.1) | 10 (71.4) | 0.142 |
| β2-microglobulin, ng/mL; mean ± SD | 3,081±2,118 | 3,531±1,752 | 0.527 |
| Albumin, g/dL; mean ± SD | 3.4±0.8 | 3.1±0.9 | 0.359 |
| White cell count, ×109/L; mean ± SD | 7.6±3.1 | 7.0±2.7 | 0.493 |
| Hemoglobin, g/dL; mean ± SD | 11.8±2.2 | 11.8±2.3 | 0.920 |
| Platelet count, ×109/L; mean ± SD | 235±107 | 205±88 | 0.293 |
| IPId score; mean ± SD | 2.19±1.44 | 2.60±1.24 | 0.307 |
| NCCN IPIe score; mean ± SD | 3.38±1.85 | 4.40±1.72 | 0.053 |
| NCCN IPI high-intermediate & high risk; n (%) | 30 (43.5) | 11 (73.3) | 0.047* |
| Extranodal involvementf; n (%) | 38 (52.1) | 7 (43.8) | 0.547 |
| Cell of origin; n (%) | 0.121 | ||
| Germinal center B-cell like | 25 (39.1) | 2 (13.3) | |
| Non-germinal center B-cell | 39 (60.9) | 13 (86.7) | |
a, ECOG performance status; b, BMI: body mass index; c, LDH: lactate dehydrogenase; d, IPI: international prognostic index; e, NCCN-IPI: National Comprehensive Cancer Network International Prognostic Index; f, extranodal involvement of either BM, CNS, GI/liver, or lung. *,P value with significance.
Table S2
| Variables | MYC | P | |
|---|---|---|---|
| <40 (N=81) | ≥40 (N=8) | ||
| Age in years; mean ± SD | 64.4±16.5 | 62.7±17.6 | 0.787 |
| Males (%) | 48 (59.3) | 4 (50.0) | 0.714 |
| Performance status 0-1a; n (%) | 60 (75.9) | 5 (62.5) | 0.411 |
| BMIb, kg/m2; mean ± SD | 23.8±4.6 | 24.8±3.4 | 0.554 |
| BMI ≥27 kg/m2; n (%) | 12 (16.2) | 3 (37.5) | 0.157 |
| LDHc level in U/L; mean ± SD | 253±249 | 488±699 | 0.411 |
| LDHc level higher than normal (%) | 39 (52.0) | 3 (42.9) | 0.709 |
| β2-microglobulin, ng/mL; mean ± SD | 2,987±2,043 | 4,355±1,602 | 0.149 |
| Albumin, g/dL; mean ± SD | 3.4±0.8 | 2.9±0.5 | 0.205 |
| White cell count, ×109/L; mean ± SD | 7.5±3.0 | 6.6±3.5 | 0.432 |
| Hemoglobin, g/dL; mean ± SD | 11.9±2.2 | 10.8±1.3 | 0.162 |
| Platelet count, ×109/L; mean ± SD | 232±107 | 181±84 | 0.190 |
| IPId score; mean ± SD | 2.23±1.40 | 2.57±1.51 | 0.544 |
| NCCN IPIe score; mean ± SD | 3.43±1.80 | 4.43±1.90 | 0.168 |
| NCCN IPI high-intermediate & high risk; n (%) | 35 (45.5) | 5 (71.4) | 0.250 |
| Extranodal involvementf; n (%) | 36 (44.4) | 6 (75.0) | 0.142 |
| Cell of origin; n (%) | 0.264 | ||
| Germinal center B-cell like | 25 (34.7) | 1 (12.5) | |
| Non-germinal center B-cell | 47 (65.3) | 7 (87.5) | |
a, ECOG performance status; b, BMI: body mass index; c, LDH: lactate dehydrogenase; d, IPI: international prognostic index; e, NCCN-IPI: National Comprehensive Cancer Network International Prognostic Index; f, extranodal involvement of either BM, CNS, GI/liver, or lung.
Acknowledgments
The authors appreciate the assistance in data collection by Miss Pei-Wen Tsai and Miss I-Shan Chen. We thank our colleague from the Tissue Bank of Chang Gung Memorial Hospital, Chiayi, for their help in archived tissue retrieval as well as processing and preparation of microarray slides.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1362
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1362
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1362). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patients included in the study all signed an informed consent stating their permission for the use of the leftover tumor samples stored in the bio-bank of our institute. The study was approved by the Institutional Review Board of Chang-Gung Memorial Hospital (Taiwan) (IRB number: 201600159B0C601) and conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040-5. [Crossref] [PubMed]
- International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987-94. [Crossref]
- Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837-42. [Crossref] [PubMed]
- Mian M, Marcheselli L, Rossi A, et al. A diachronic-comparative analysis for the identification of the most powerful prognostic index for localized diffuse large B-cell lymphoma. Ann Oncol 2014;25:2398-404. [Crossref] [PubMed]
- Melchardt T, Troppan K, Weiss L, et al. A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and β2 -microglobulin. Br J Haematol 2015;168:239-45. [Crossref] [PubMed]
- Huang CE, Chen YY, Lu CH, et al. Validation of an enhanced International Prognostic Index (NCCN-IPI) in an Asian cohort of patients with diffuse large B cell lymphoma. Ann Hematol 2015;94:1063-5. [Crossref] [PubMed]
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82. [Crossref] [PubMed]
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11. [Crossref] [PubMed]
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937-47. [Crossref] [PubMed]
- Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol 2014;11:12-23. [Crossref] [PubMed]
- Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol 2015;16:e555-67. [Crossref] [PubMed]
- Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3452-9. [Crossref] [PubMed]
- Wang XJ, Medeiros LJ, Lin P, et al. MYC cytogenetic status correlates with expression and has prognostic significance in patients with MYC/BCL2 protein double-positive diffuse large B-cell lymphoma. Am J Surg Pathol 2015;39:1250-8. [Crossref] [PubMed]
- Swerdlow SH. Diagnosis of 'double hit' diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program 2014;2014:90-9. [Crossref] [PubMed]
- Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist 2010;15:1083-101. [Crossref] [PubMed]
- Larsson SC, Wolk A. Obesity and risk of non-Hodgkin's lymphoma: a meta-analysis. Int J Cancer 2007;121:1564-70. [Crossref] [PubMed]
- Kane E, Skibola CF, Bracci PM, et al. Non-Hodgkin Lymphoma, Body Mass Index, and Cytokine Polymorphisms: A Pooled Analysis from the InterLymph Consortium. Cancer Epidemiol Biomarkers Prev 2015;24:1061-70. [Crossref] [PubMed]
- Geyer SM, Morton LM, Habermann TM, et al. Smoking, alcohol use, obesity, and overall survival from non-Hodgkin lymphoma: a population-based study. Cancer 2010;116:2993-3000. [Crossref] [PubMed]
- Jones JA, Fayad LE, Elting LS, et al. Body mass index and outcomes in patients receiving chemotherapy for intermediate-grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma 2010;51:1649-57. [Crossref] [PubMed]
- Carson KR, Bartlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J Clin Oncol 2012;30:3217-22. [Crossref] [PubMed]
- Weiss L, Melchardt T, Habringer S, et al. Increased body mass index is associated with improved overall survival in diffuse large B-cell lymphoma. Ann Oncol 2014;25:171-6. [Crossref] [PubMed]
- Hong F, Habermann TM, Gordon LI, et al. The role of body mass index in survival outcome for lymphoma patients: US intergroup experience. Ann Oncol 2014;25:669-74. [Crossref] [PubMed]
- Ganti A, Liu W, Luo S, et al. Impact of body mass index on incidence of febrile neutropenia and treatment-related mortality in United States veterans with diffuse large B-cell lymphoma receiving rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. Br J Haematol 2014;167:699-702. [Crossref] [PubMed]
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244. [Crossref] [PubMed]
- Lu CH, Lee KF, Chen CC, et al. Clinical characteristics and treatment outcome in a Taiwanese population of patients with Epstein-Barr virus-positive diffuse large B-cell lymphoma. Jpn J Clin Oncol 2014;44:1164-71. [Crossref] [PubMed]
- Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 2014;123:1214-7. [Crossref] [PubMed]
- Li HJ, Che XM, Zhao W, et al. Diet-induced obesity promotes murine gastric cancer growth through a nampt/sirt1/c-myc positive feedback loop. Oncol Rep 2013;30:2153-60. [Crossref] [PubMed]
- Picon-Ruiz M, Pan C, Drews-Elger K, et al. Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/miR-302b-Mediated Malignant Progression. Cancer Res 2016;76:491-504. [Crossref] [PubMed]
- Perera CN, Chin HG, Duru N, et al. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol 2008;199:221-33. [Crossref] [PubMed]
- Chen C, Chang YC, Liu CL, et al. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat 2006;98:121-32. [Crossref] [PubMed]
- Walford GA, Gustafsson S, Rybin D, et al. Genome-wide association study of the modified Stumvoll Insulin Sensitivity Index identifies BCL2 and FAM19A2 as novel insulin sensitivity loci. Diabetes 2016;65:3200-11. [Crossref] [PubMed]
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. [Crossref] [PubMed]


