
The prognosis of small-sized non-small cell lung cancer with visceral pleural invasion after sublobar resection
Introduction
The standard surgical treatment of stage I non-small cell lung cancer (NSCLC) is lobectomy (1). Recently, however, sublobar resection has been increasingly applied for the treatment of stage I NSCLC. In particular, as more people undergo regular checkups, the early detection of lung cancer is increasing, and the detection of less invasive lung cancer is also increasing. The discovery of ground-glass opacity (GGO) nodules on chest computed tomography (CT) has also increased, and sublobar resection has been actively performed for the treatment of GGO. In the case of lung cancer presenting as GGO on CT scan, the prognosis of sublobar resection is known to be acceptable. There are many studies that support these results (2-5). In addition to the increasing frequency of sublobar resection of GGO-dominant tumors, studies have been conducted to demonstrate the efficacy of sublobar resection for solid-dominant tumors of 2 cm or less. Two important randomized controlled trials (JCOG0802 and CALGB140503) of sublobar resection for solid-dominant tumors are being conducted and will produce results in a few years (6-8). The results of these studies will clearly demonstrate the efficacy of sublobar resection in small solid-dominant lung cancer.
There are many studies to evaluate the prognosis of sublobar resection for stage IA NSCLC of 2 cm or less (9-11). However, there have been no studies on the prognosis of sublobar resection in stage IB NSCLC of 2 cm or less. Indeed, patients with clinical stage IA lung cancer of <2 cm who underwent sublobar resection were sometimes found to have visceral pleural invasion in the postoperative pathological report. If such a result is obtained, it is difficult to determine whether proper treatment was achieved by sublobar resection alone because upstaging had occurred from stage IA to IB. In such cases, the question is whether additional completion lobectomy should be performed immediately.
The eighth edition of the TNM staging system has substantial revisions compared to the seventh edition staging system (12-14). In particular, the criteria for measuring tumor size were changed to measure the size of invasive components rather than the overall tumor size. Because of these changes in the staging system, it is necessary to apply a new staging system to determine the prognosis of sublobar resection at any stage of NSCLC.
The purpose of this study was to evaluate the prognosis of patients with small-sized (invasive component size ≤2 cm) stage IB NSCLC after sublobar resection. In those cases, sublobar resection was performed initially for the treatment of small (invasive component size ≤2 cm) tumors, and, postoperatively, histopathological findings revealed the presence of visceral pleural invasion. Through this research, we wanted to find out whether the additional completion lobectomy should be done immediately in such cases. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-1995).
Methods
Patients
From January 2010 to December 2018, 1994 patients underwent curative resection of NSCLC at a tertiary hospital in South Korea. Of those patients, 298 patients were diagnosed as having stage IB NSCLC according to the eighth edition of TNM staging system. Patients who underwent neo-adjuvant chemotherapy or adjuvant chemotherapy were excluded from this study. Patients who had residual tumor in the lung or in the resected margins were also excluded. Patients with tumors larger than 2 cm of invasive component size who underwent sublobar resection were also excluded. To reduce the selection bias, all data were obtained from consecutive patient data. Finally, 227 consecutive patients were reviewed retrospectively. The patients were divided into two groups: the sublobar resection group (n=21) and the lobectomy group (n=206). The sublobar resection group included only tumors ≤2 cm of invasive component size. The clinicopathological characteristics were analyzed in the two groups. The comparison of prognosis was conducted in the two groups. We also conducted a study comparing the prognosis of sublobar resection and lobectomy in tumors ≤2 cm of invasive component size. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea (referral number: KC20RASI0020) and individual consent was waived in this retrospective study.
Surgical procedures
Patients diagnosed with clinical stage I lung cancer on chest CT scan and combination positron emission tomography (PET) and CT scan were eligible for surgical treatment. The treatment of choice for stage I NSCLC is lobectomy with mediastinal lymph node dissection. However, in patients with GGO or small solid peripheral nodules near the visceral pleura, sublobar resection is also considered. The surgical procedure was selected depending on the surgeon’s preference or the patient’s decision, and in the case of high-risk patients with cardiopulmonary disease, sublobar resection was usually performed. Sublobar resection consists of wedge resection and segmentectomy. In most cases, a sufficient resection margin was obtained, in which the margin length was greater than the tumor diameter.
Histological evaluation and restaging
All pathology slides and pathology reports were reviewed. Pathology reports included tumor size, tumor location, nodal status, pleural invasion, lymphatic invasion, and vascular invasion. Visceral pleural invasion was defined as a tumor extending beyond the elastic layer. Lymphatic invasion or vascular invasion was defined as tumor cells present in the lymphatic vessel or vascular lumen. TNM staging was based on the eighth edition of the TNM staging system of lung cancer (14). To reclassify the T category according to the eighth edition, tumor size was remeasured by the pathologist at the greatest diameter of the invasive component on a histopathological preparation (13). Cases where the invasive component size was ≤2 cm were defined as small-sized NSCLC.
Statistical analysis
The clinicopathological characteristics of the sublobar resection group and lobectomy group were compared. A Student t-test or Wilcoxon rank-sum test was used for continuous variables, and the χ2 test or Fisher exact test was applied for categorical variables. The Kaplan-Meier method was used to analyze data collected from the interval between the time of operation and the time of the last follow-up visit. Recurrence-free survival (RFS) rates and overall survival (OS) rates were estimated by the Kaplan-Meier method. Survival of each group was compared by log-rank test. The Cox proportional hazards model was used in a multivariate analysis to determine the risk factor of recurrence and death for all the study patients. The variables with a P value <0.1 by univariate analysis were entered into a multivariate analysis. A P value <0.05 was considered statistically significant.
Results
Comparison of sublobar resection and lobectomy in all study patients
Table 1 shows the comparison of clinical and pathological characteristics between the sublobar resection group and the lobectomy group. There was no statistical difference in clinical characteristics between the two groups except maximum standardized uptake value (SUVmax) on PET. The mean SUVmax of the lobectomy group was greater than that of the sublobar resection group (7.2 vs. 3.5, P<0.001). In pathological characteristics, most factors were also not different between the two groups except tumor size and the presence of visceral pleural invasion. Tumor size and invasive component size were larger in the lobectomy group. Visceral pleural invasive was present in 100% of the sublobar resection group, but in only 72.3% of the lobectomy group (P=0.006).
Table 1
| Variables | Sublobar resection group (n=21) | Lobectomy group (n=206) | P value |
|---|---|---|---|
| Age (± SD) | 67.5 (±10.1) | 66.9 (±10.8) | 0.799 |
| Gender, n (%) | 0.820 | ||
| Male | 11 (52.4) | 115 (55.8) | |
| Female | 10 (47.6) | 91 (44.2) | |
| Current or former smoker, n (%) | 9 (42.9) | 96 (46.6) | 0.821 |
| Serum CEA level (ng/mL) (± SD) | 4.1 (±5.7) | 3.0 (±3.3) | 0.449 |
| SUVmax (± SD) | 3.5 (±2.4) | 7.2 (±4.2) | <0.001 |
| Involved lobe, n (%) | 0.402 | ||
| Right upper | 7 (33.3) | 66 (32.0) | |
| Right middle | 0 | 23 (11.2) | |
| Right lower | 7 (33.3) | 49 (23.8) | |
| Left upper | 3 (14.3) | 42 (20.4) | |
| Left lower | 4 (19.0) | 26 (12.6) | |
| Surgical approach, n (%) | 0.702 | ||
| VATS | 20 (95.2) | 185 (89.8) | |
| Open thoracotomy | 1 (4.8) | 21 (10.2) | |
| Surgical procedures | <0.001 | ||
| Wedge resection | 14 (66.7) | 0 | |
| Segmentectomy | 7 (33.3) | 0 | |
| Lobectomy | 0 | 201 (97.6) | |
| Bilobectomy | 0 | 5 (2.4) | |
| Intraoperative lymph node evaluation, n (%) | <0.001 | ||
| No mediastinal node dissection | 4 (19.0) | 6 (2.9) | |
| Systematic nodal dissection | 5 (23.8) | 162 (78.6) | |
| Selective nodal dissection | 12 (57.1) | 38 (18.4) | |
| Postoperative complications, n (%) | 1 (4.8) | 43 (20.9) | 0.086 |
| Operative mortality, n (%) | 0 | 2 (1.0) | 1.000 |
| Postoperative hospital stay (days) (± SD) | 6.2 (±3.2) | 8.3 (±8.8) | 0.276 |
| Histology, n (%) | 0.196 | ||
| Adenocarcinoma | 19 (90.5) | 147 (71.4) | |
| Squamous cell carcinoma | 1 (4.8) | 40 (19.4) | |
| Others | 1 (4.8) | 19 (9.2) | |
| Total tumor size (cm) (± SD) | 1.6 (±0.4) | 2.8 (±0.9) | <0.001 |
| Invasive component size (cm) (± SD) | 1.5 (±0.3) | 2.6 (±0.9) | <0.001 |
| Location, n (%) | 0.140 | ||
| Central | 0 | 24 (11.7) | |
| Peripheral | 21 (100.0) | 182 (88.3) | |
| Histological tumor grade, n (%) | 0.138 | ||
| Well differentiated carcinoma | 8 (38.1) | 40 (19.4) | |
| Moderately differentiated carcinoma | 9 (42.9) | 110 (53.4) | |
| Poorly differentiated carcinoma | 4 (19.0) | 56 (27.2) | |
| Number of dissected lymph nodes (± SD) | 6.3 (±8.9) | 14.5 (±7.5) | <0.001 |
| Visceral pleural invasion, n (%) | 21 (100.0) | 149 (72.3) | 0.006 |
| Lymphatic invasion, n (%) | 10 (47.6) | 104 (50.5) | 0.823 |
| Vascular invasion, n (%) | 6 (28.6) | 49 (23.8) | 0.790 |
SD, standard deviation; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; VATS, video-assisted thoracoscopic surgery.
The median follow-up period for all study patients was 1,348 (range, 33–3,443) days, and 46 patients had recurrence (Table 2). The 5-year RFS rate was 80.7% after sublobar resection and 73.4% after lobectomy (Figure 1A). The 5-year OS rate was 87.3% after sublobar resection and 84.8% after lobectomy (Figure 1B). Both RFS and OS were not statistically different between the sublobar resection group and the lobectomy group (P=0.349 and P =0.503, respectively). The univariate and multivariate analyses using a Cox proportional hazards model were conducted to find out the risk factor for recurrence (Table 3). Sublobar resection was not a significant risk factor for recurrence in the univariate analysis. Specific variables identified as significant (P<0.1) by univariate analysis included SUVmax, involved lobes, histological tumor grade, and lymphatic invasion. When these variables were entered into the multivariate model, only histological tumor grade was a significant risk factor for recurrence of all study patients (P=0.017).
Table 2
| Variables | Sublobar resection group (n=21) | Lobectomy group (n=206) | P value |
|---|---|---|---|
| Sites of recurrence | 0.654 | ||
| Locoregional recurrence | 3 | 21 | |
| Distant recurrence | 0 | 13 | |
| Both | 0 | 9 |
Locoregional, recurrence within ipsilateral hemithorax including pleura and mediastinal lymph nodes. Both, locoregional recurrence + distant recurrence.
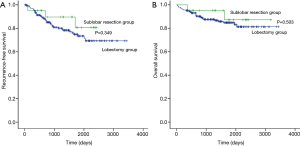
Table 3
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 0.992 | 0.965–1.019 | 0.547 |
| Gender (male) | 1.013 | 0.564–1.820 | 0.966 |
| Smoker | 1.114 | 0.613–2.024 | 0.723 |
| Serum CEA level | 1.027 | 0.955–1.105 | 0.469 |
| SUVmax | 1.080 | 1.018–1.147 | 0.011 |
| Involved lobe | 0.089 | ||
| Right upper (reference) | 1 | ||
| Right middle | 1.069 | 0.381–3.000 | 0.899 |
| Right lower | 0.758 | 0.314–1.829 | 0.537 |
| Left upper | 2.051 | 0.974–4.318 | 0.059 |
| Left lower | 0.628 | 0.205–1.928 | 0.416 |
| VATS | 1.261 | 0.450–3.533 | 0.659 |
| Sublobar resection | 0.575 | 0.178–1.857 | 0.355 |
| Intraoperative lymph node evaluation | 0.394 | ||
| No mediastinal node dissection (reference) | 1 | ||
| Systematic nodal dissection | 0.608 | 0.215–1.722 | 0.349 |
| Selective nodal dissection | 0.436 | 0.131–1.450 | 0.176 |
| Histology | 0.529 | ||
| Adenocarcinoma (reference) | 1 | ||
| Squamous cell carcinoma | 0.948 | 0.419–2.142 | 0.897 |
| Others | 1.686 | 0.658–4.321 | 0.277 |
| Total tumor size | 0.999 | 0.719–1.388 | 0.995 |
| Invasive component size | 1.054 | 0.761–1.459 | 0.753 |
| Central location | 0.942 | 0.371–2.389 | 0.900 |
| Histological tumor grade | 0.004 | ||
| Well differentiated carcinoma (reference) | 1 | ||
| Moderately differentiated carcinoma | 2.869 | 0.994–8.283 | 0.051 |
| Poorly differentiated carcinoma | 5.783 | 1.931–17.320 | 0.002 |
| Number of dissected lymph nodes | 0.999 | 0.964–1.036 | 0.960 |
| Visceral pleural invasion | 1.293 | 0.623–2.684 | 0.491 |
| Lymphatic invasion | 2.180 | 1.183–4.018 | 0.012 |
| Vascular invasion | 1.397 | 0.720–2.708 | 0.323 |
| Lymphovascular invasion | 2.165 | 1.163–4.029 | 0.015 |
| Multivariate analysis | |||
| SUVmax | 1.007 | 0.938–1.083 | 0.840 |
| Lobe | 0.097 | ||
| Right upper (reference) | 1 | ||
| Right middle | 1.271 | 0.452–3.573 | 0.649 |
| Right lower | 0.699 | 0.274–1.780 | 0.453 |
| Left upper | 2.128 | 0.999–4.532 | 0.050 |
| Left lower | 0.685 | 0.193–2.434 | 0.558 |
| Histological tumor grade | 0.017 | ||
| Well differentiated carcinoma (reference) | 1 | ||
| Moderately differentiated carcinoma | 3.043 | 0.882–10.499 | 0.078 |
| Poorly differentiated carcinoma | 6.408 | 1.640–25.043 | 0.008 |
| Lymphatic invasion | 1.344 | 0.682–2.651 | 0.393 |
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; VATS, video-assisted thoracoscopic surgery.
Reason for sublobar resection
Table 4 shows the reasons for sublobar resection in 21 patients. Intentional sublobar resection was performed in 10 patients. Of those 10 patients, eight tumors were part-solid GGOs and two tumors were peripheral solid nodules on chest CT. Five patients underwent sublobar resection because of underlying cardiopulmonary disease. Four patients underwent sublobar resection due to previous lung surgery history. One patient had a hematologic malignancy and one patient was 86 years old.
Table 4
| Reasons | N (%) |
|---|---|
| Intentional sublobar resection | 10 (47.6) |
| Underlying cardiopulmonary disease | 5 (23.8) |
| Previous lung operation | 4 (19.0) |
| Underlying other malignant disease | 1 (4.8) |
| Old age | 1 (4.8) |
Comparison of sublobar resection and lobectomy in small-sized stage IB NSCLC
Of all study patients, 84 patients had small-sized (invasive component size ≤2 cm) stage IB NSCLC. We compared the prognosis of sublobar resection and lobectomy in the same size group. Table 5 shows the comparison of clinical and pathological characteristics between the sublobar resection group and the lobectomy group. There was no statistical difference in clinical and pathological characteristics between the two groups.
Table 5
| Variables | Sublobar resection group (n=21) | Lobectomy group (n=63) | P value |
|---|---|---|---|
| Age (± SD) | 67.5 (±10.1) | 64.0 (±11.3) | 0.211 |
| Gender, n (%) | 0.613 | ||
| Male | 11 (52.4) | 27 (42.9) | |
| Female | 10 (47.6) | 36 (57.1) | |
| Current or former smoker, n (%) | 9 (42.9) | 22 (34.9) | 0.604 |
| Serum CEA level (ng/mL) (± SD) | 4.1 (±5.7) | 2.4 (±2.4) | 0.216 |
| SUVmax (± SD) | 3.5 (±2.4) | 4.6 (±2.7) | 0.121 |
| Involved lobe, n (%) | 0.421 | ||
| Right upper | 7 (33.3) | 18 (28.6) | |
| Right middle | 0 | 7 (11.1) | |
| Right lower | 7 (33.3) | 14 (22.2) | |
| Left upper | 3 (14.3) | 15 (23.8) | |
| Left lower | 4 (19.0) | 9 (14.3) | |
| Surgical approach, n (%) | 1.000 | ||
| VATS | 20 (95.2) | 58 (92.1) | |
| Open thoracotomy | 1 (4.8) | 5 (7.9) | |
| Surgical procedures, n (%) | <0.001 | ||
| Wedge resection | 14 (66.7) | 0 | |
| Segmentectomy | 7 (33.3) | 0 | |
| Lobectomy | 0 | 62 (98.4) | |
| Bilobectomy | 0 | 1 (1.6) | |
| Intraoperative lymph node evaluation, n (%) | <0.001 | ||
| No mediastinal node dissection | 4 (19.0) | 2 (3.2) | |
| Systematic nodal dissection | 5 (23.8) | 44 (69.8) | |
| Selective nodal dissection | 12 (57.1) | 17 (27.0) | |
| Postoperative complications, n (%) | 1 (4.8) | 8 (12.7) | 0.439 |
| Operative mortality, n (%) | 0 | 0 | |
| Postoperative hospital stay (days) (± SD) | 6.2 (±3.2) | 6.2 (±3.0) | 0.959 |
| Histology, n (%) | 1.000 | ||
| Adenocarcinoma | 19 (90.5) | 56 (88.9) | |
| Squamous cell carcinoma | 1 (4.8) | 4 (6.3) | |
| Others | 1 (4.8) | 3 (4.8) | |
| Total tumor size (± SD) | 1.6 (±0.4) | 1.9 (±0.6) | 0.034 |
| Invasive component size (± SD) | 1.5 (±0.3) | 1.5 (±0.3) | 0.600 |
| Location, n (%) | 0.570 | ||
| Central | 0 | 3 (4.8) | |
| Peripheral | 21 (100.0) | 60 (95.2) | |
| Histological tumor grade, n (%) | 0.741 | ||
| Well differentiated carcinoma | 8 (38.1) | 19 (30.2) | |
| Moderately differentiated carcinoma | 9 (42.9) | 34 (54.0) | |
| Poorly differentiated carcinoma | 4 (19.0) | 10 (15.9) | |
| Number of dissected lymph nodes (± SD) | 6.3 (±8.9) | 12.6 (±6.2) | 0.001 |
| Visceral pleural invasion, n (%) | 21 (100.0) | 61 (96.8) | 1.000 |
| Lymphatic invasion, n (%) | 10 (47.6) | 29 (46.0) | 1.000 |
| Vascular invasion, n (%) | 6 (28.6) | 10 (15.9) | 0.213 |
NSCLC, non-small cell lung cancer; SD, standard deviation; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; VATS, video-assisted thoracoscopic surgery.
The median follow-up period for patients with small-sized stage IB NSCLC was 1,401 (range, 335–3,443) days, and 18 patients had recurrence (Table 6). The 5-year RFS rate was 80.7% after sublobar resection and 72.3% after lobectomy (Figure 2A). The 5-year OS rate was 87.3% after sublobar resection and 91.2% after lobectomy (Figure 2B). There was no difference in RFS and OS between the sublobar resection group and the lobectomy group (P=0.417 and P=0.956, respectively). We conducted Cox proportional hazards model to find out the risk factor for recurrence (Table 7). In the univariate analysis, sublobar resection was not a significant risk factor for recurrence in patients with small-sized stage IB NSCLC. SUVmax, histological tumor grade, and lymphatic invasion were significant variables (P<0.1) in the univariate analysis, and these factors were entered into the multivariate analysis. However, no variables were identified as significant risk factors in the multivariate analysis.
Table 6
| Variables | Sublobar resection group (n=21) | Lobectomy group (n=63) | P value |
|---|---|---|---|
| Sites of recurrence | 0.824 | ||
| Locoregional recurrence | 5 | 8 | |
| Distant recurrence | 0 | 4 | |
| Both | 0 | 1 |
Locoregional, recurrence within ipsilateral hemithorax including pleura and mediastinal lymph nodes. Both, locoregional recurrence + distant recurrence. NSCLC, non-small cell lung cancer.
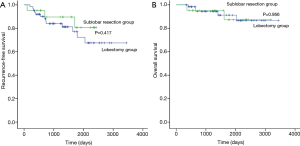
Table 7
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 0.983 | 0.939–1.030 | 0.479 |
| Gender (male) | 1.028 | 0.382–2.764 | 0.957 |
| Smoker | 1.037 | 0.352–3.055 | 0.947 |
| Serum CEA level | 1.037 | 0.929–1.157 | 0.521 |
| SUVmax | 1.254 | 1.059–1.485 | 0.009 |
| Lobe | 0.306 | ||
| Right upper (reference) | 1 | ||
| Right middle | 2.005 | 0.182–22.141 | 0.570 |
| Right lower | 2.677 | 0.490–14.639 | 0.256 |
| Left upper | 5.460 | 1.099–27.133 | 0.038 |
| Left lower | 2.551 | 0.424–15.341 | 0.306 |
| VATS | 1.030 | 0.136–7.819 | 0.977 |
| Sublobar resection | 0.597 | 0.170–2.102 | 0.422 |
| Intraoperative lymph node evaluation | 0.573 | ||
| No mediastinal node dissection (reference) | 1 | ||
| Systematic nodal dissection | 0.683 | 0.149–3.139 | 0.624 |
| Selective nodal dissection | 0.424 | 0.077–2.328 | 0.324 |
| Histology | 0.990 | ||
| Adenocarcinoma (reference) | 1 | ||
| Squamous cell carcinoma | 1.153 | 0.115–6.466 | 0.891 |
| Others | 0 | 0 | 0.983 |
| Total tumor size | 1.135 | 0.481–2.677 | 0.773 |
| Invasive component size | 1.942 | 0.448–8.422 | 0.375 |
| Central location | 3.563 | 0.455–27.881 | 0.226 |
| Histological tumor grade | 0.078 | ||
| Well differentiated carcinoma (reference) | 1 | ||
| Moderately differentiated carcinoma | 2.635 | 0.704–9.862 | 0.150 |
| Poorly differentiated carcinoma | 6.087 | 1.269–29.199 | 0.024 |
| Number of dissected lymph nodes | 1.003 | 0.941–1.068 | 0.932 |
| Lymphatic invasion | 3.775 | 1.216–11.719 | 0.022 |
| Vascular invasion | 0.249 | 0.033–1.886 | 0.178 |
| Multivariate analysis | |||
| SUVmax | 1.161 | 0.948–1.423 | 0.150 |
| Histological tumor grade | 0.347 | ||
| Well differentiated carcinoma (reference) | 1 | ||
| Moderately differentiated carcinoma | 2.068 | 0.397–10.766 | 0.388 |
| Poorly differentiated carcinoma | 4.221 | 0.577–30.869 | 0.156 |
| Lymphatic invasion | 1.548 | 0.394–6.084 | 0.532 |
NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; SUVmax, maximum standardized uptake value; VATS, video-assisted thoracoscopic surgery.
Discussion
Sublobar resection is not usually recommended for the treatment of stage IB NSCLC. However, because visceral pleural invasion is diagnosed only after surgery, it is sometimes diagnosed as postoperative stage IB when sublobar resection was performed for small peripheral nodules. In this study, sublobar resection for small-sized (invasive component size ≤2 cm) stage IB NSCLC had comparable prognosis with lobectomy. Firstly, we compared the prognosis between patients with small-sized stage IB NSCLC who underwent sublobar resection and patients with any stage IB NSCLC who underwent lobectomy. All patients were consecutive patients in the same hospital and underwent the same treatment protocols; moreover, both groups were well matched in clinicopathological characteristics except SUVmax and invasive component size. Thus, we then compared the prognosis of sublobar resection and lobectomy in patients with small-sized stage IB NSCLC. In this analysis, all clinicopathological characteristics were well matched and RFS and OS rate were not different in the statistical analysis. Furthermore, sublobar resection was not a risk factor for recurrence in two multivariate analyses in this study. Therefore, we concluded that sublobar resection for small-sized stage IB NSCLC had the same prognosis as lobectomy. In other words, these patients may not need an additional completion lobectomy performed immediately.
After implementation of the eighth revision of the TNM classification of NSCLC, the composition of the tumors included in the stage IB classification was changed. Most importantly, the requirement for measuring tumor size was changed. Determination of the T stage in the eighth revision is based only on the maximum dimension of the invasive component and excludes the lepidic component (13,15). The size range of the T2a descriptor was also reduced from 3 to 5 cm to 3 to 4 cm. Therefore, the tumor characteristics for the seventh edition stage IB NSCLC were changed in the eighth edition. Because of these changes, we thought that if the stage-based postoperative prognosis is studied, in all cases it is necessary to restudy after applying the eighth edition of TNM staging. This study is also the first to study the prognosis of sublobar resection of stage IB by applying the eighth edition of the TNM staging system.
Sublobar resections are usually performed for small-sized peripheral tumors in our institution. Particularly, patients with GGO tumors (consolidation: tumor ratio <0.5) were candidates for intentional sublobar resection. Ten patients (47.6%) underwent intentional sublobar resection in this study. On the other hand, seven patients underwent sublobar resection because of a poor general health condition (underlying cardiopulmonary disease, underlying hematologic malignant disease, and old age). Four patients underwent sublobar resection because of previous contralateral lung surgery. Although the sublobar resection group was not homogenous and the decisions for performing sublobar resection were varied, all study data were collected from consecutive patients who underwent curative surgery at one institution and clinicopathological characteristics were not different between the sublobar resection group and the lobectomy group. Thus, the findings of this study are considered meaningful.
The tumors of the sublobar resection group were located near the visceral pleura. Those tumors all invaded the visceral pleura, so their stage was upstaged from clinical T1a–b to pathological T2a. The tumors were all attached to the visceral pleura, making wedge resection and segmentectomy relatively uncomplicated to perform. It was also easy to ensure sufficient margins after sublobar resection. Studies have shown that the resection margin should be at least the tumor size when sublobar resection is performed (16-18). In this study, not only was the resection margin longer than the tumor size, but more sufficient lung parenchyma was removed. In the case of tumors adjacent to the visceral pleura, the resection margin can be sufficiently excised even by sublobar resection. Therefore, it may be assumed that sublobar resection might be as effective as lobectomy even for peripheral small-sized stage IB.
There have been few studies analyzing the prognosis of sublobar resection in stage IB NSCLC. This is because, at stage IB, it is generally accepted that lobectomy should be performed. Our previous study reported that sublobar resection for small-sized (≤2 cm) NSCLC with visceral pleural invasion or lymphatic invasion had a similar prognosis as lobectomy (19). Of course, the previous study yielded similar results to the current study; however, the previous study included patients with lymphatic invasion, while the current study included only patients with visceral pleural invasion. Among the cases of visceral pleural and lymphatic invasion, only visceral pleural invasion is the upstaging factor. This is because only visceral pleural invasion can upstage small lung cancers, leading to stage IB. The previous study was based on the seventh edition TNM staging system, while the current study adopted the eighth edition of the TNM staging system. Furthermore, previous studies have included large numbers of patients before 2010; however, this study consists only of data since 2010. This study, which contains relatively new data and adopts the new TNM staging system, is expected to predict more accurate results than previous studies of sublobar resection for small-sized stage IB NSCLC.
In this study, the choice of wedge resection or segmentectomy depended on the adequate resection margin. Wedge resection was usually considered first, but segmentectomy was performed if the width of the resection margin appeared to be shorter than the tumor size. The segmental artery and bronchus were divided separately, and then the segmental plane was divided by using endostaplers. So, the basic surgical techniques of wedge resection and segmentectomy were similar in the author’s institution. Moreover, due to the small number of segmentectomy cases, the study was not conducted by classifying wedge resection and segmentectomy. Further studies that include data from larger cohorts may validate these conclusions and provide more refined results.
This study has a few limitations. First, it was a retrospective review. Second, we obtained data from a single institution, and the sample size was relatively small from which to generalize our results. However, this study examined data from surgical patients treated with a standardized protocol at an institution, a tertiary hospital in Korea. Furthermore, a very detailed analysis was possible because of the comprehensive information stored in the electronic medical record. We also had no problem applying the new staging system using pathology slides. We believe that our data will be useful as the basis for future investigations. A prospective randomized controlled study should be performed to validate our results. Finally, patients with a short follow-up period were included in this study. However, most patients with NSCLC are known to have disease recurrence within a 2-year postoperative period (20), and early recurrence has been shown to be an accurate reflection of long-term outcomes (21).
In conclusion, the prognosis of sublobar resection in patients with small-sized (≤2 cm) stage IB NSCLC was comparable with lobectomy. Thus, additional completion lobectomy is not essential in this setting, despite postoperative upstaging from T1 to T2a. Further research through multicenter randomized controlled trials may more accurately depict patient outcomes.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1995
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1995
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-1995
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1995). Dr. YM reports grans from National Research Foundation of Korea (NRF), during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea and individual consent was waived (referral number: KC20RASI0020).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6; discussion 636. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. The prognosis of invasive adenocarcinoma presenting as ground-glass opacity on chest computed tomography after sublobar resection. J Thorac Dis 2017;9:3782-92. [Crossref] [PubMed]
- Moon Y, Lee KY, Moon SW, et al. Sublobar resection margin width does not affect recurrence of clinical N0 non-small cell lung cancer presenting as GGO-predominant nodule of 3 cm or less. World J Surg 2017;41:472-9. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after sublobar resection for early-stage lung cancer: methodological obstacles in comparing the efficacy to lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Prognosis after wedge resection in patients with 8th edition TNM stage IA1 and IA2 non-small cell lung cancer. J Thorac Dis 2019;11:2361-72.
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:990-1003.
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY. The effect of resection margin distance and invasive component size on recurrence after sublobar resection in patients with small (≤2 cm) lung adenocarcinoma. World J Surg 2020;44:990-7. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Margin width of resected lepidic lung cancer does not affect recurrence after sublobar resection. World J Surg 2018;42:1449-57. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Prognosis after sublobar resection of small-sized non-small cell lung cancer with visceral pleural or lymphovascular invasion. World J Surg 2017;41:2769-77. [Crossref] [PubMed]
- Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thorac Surg Clin 2013;23:429-36. [Crossref] [PubMed]
- Kiankhooy A, Taylor MD, LaPar DJ, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg 2014;98:1175-83. [Crossref] [PubMed]


