
Metformin suppressed tumor necrosis factor-α-induced epithelial-mesenchymal transition in prostate cancer by inactivating the NF-κB signaling pathway
Introduction
In 2008, there were 914,000 new cases and 258,000 deaths from prostate cancer (PCa), making it the second most commonly diagnosed cancer in men (1). Despite advances in the treatment of PCa, there is no perfect effective therapy for cancer recurrence and distant metastasis (1). Therefore, it is necessary to explore the mechanisms underlying the progression and formation of distant metastases in PCa patients to promote the development of therapeutic strategies for PCa.
Metformin is an inexpensive and commonly used oral drug for diabetes mellitus. Recently, multiple studies have shown that metformin might not only reduce cancer risk but also improve cancer prognosis (2). Metformin might exert its anti-neoplastic properties via the following mechanisms: activation of AMPK; targeted damage to P53-deficient cells; downregulation of Cyclin D1 and inhibition of C-myc (3-5).
Most mortality in PCa patients is caused by metastasis. Increasing studies have revealed that epithelial-mesenchymal transition (EMT) participates in the process of tumorigenesis, metastasis, relapse and drug resistance in various kinds of cancers (6-11). EMT is characterized by the loss of the epithelial phenotype and acquisition of mesenchymal characteristics, which endow benign tumors with malignant properties and promote cancer progression to more invasive and metastatic types (6,12,13).
Tumor necrosis factor-α (TNF-α) is an inflammatory mediator which plays a pivotal role in inflammation-associated cancer (14). A high dose of TNF-α has anti-neoplastic properties and has even been used as a cytotoxic agent. However, exposure to chronic low-level TNF-α endows cancer cells with more aggressive behaviors, such as increased growth, invasion and metastasis (14). TNF-α has been shown to promote the EMT process in various cancers, including PCa, via the NF-κB signaling pathway or a GSK3β-dependent mechanism (15-20).
Recently, reports (21,22) showed that metformin decreased PCa mortality, increased PCa sensitivity to treatment, and delayed the development of castration-resistant prostate cancer (CRPC) in PCa patients. Metformin inhibited pro-neoplastic inflammatory factors, such as IL-6 and TGF-β, and induced EMT in lung adenocarcinoma and PCa (23,24). In this study, we aimed to assess the expression of TNF-α, NF-κB-P65, and E-cadherin in human prostate cancer tissues and the effect of metformin on TNF-α-induced EMT in prostate cancer PC3 cells.
Methods
Patients and specimens
Forty-six PCa and 25 benign prostatic hyperplasia (BPH) patients frozen tissue samples were obtained from the Department of Pathology, Renmin Hospital of Wuhan University, China. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (Wuhan, China) (NO.: RM2018Y018-06) and informed consent was taken from all the patients. There were no differences in age between the two groups. Among the 46 cases of PCa, 15 cases were classified as having a low Gleason score (4-7), and the other 31 cases were classified as having a high Gleason score (8-10).
Immunohistochemical analysis and evaluation
Serial sections (thickness, 5 µm) were cut from the tissue blocks, deparaffinized in xylene, and hydrated in a graded series of alcohol. Staining was then performed using the DAB chromogenic agent (Dako Corp, USA). Primary antibodies were specific against TNF-α (rabbit monoclonal, 1:50, Cell Signaling Technology Cat# 11948, RRID: AB_2687962), NF-κB-P65 (rabbit monoclonal, 1:100, Cell Signaling Technology, USA), and E-cadherin (mouse monoclonal, 1:100, Cell Signaling Technology Cat# 14472, RRID: AB_2728770). Negative control staining was performed routinely. The immunohistochemical staining results were evaluated independently by two experienced pathologists which were categorized by the semiquantitative scoring system suggested by Remmele and Stegner (25). Accordingly, the overall score of 0–1 was designated as negative (‒), 2–3 as weak (+), 4–6 as moderate (++), and >6 as strong (+++).
Cell culture
PC3 cells (ATCC Cat# CRL-7934, RRID: CVCL_0035) were cultured in RPMI-1640 medium containing 10% FBS (Gibco/Invitrogen, Australia) and 1% penicillin/streptomycin (Invitrogen) at 37 °C in a humidified incubator composed of 95% air and 5% CO2. Recombinant human TNF-α protein was purchased from PeproTech (Rocky Hill, NJ). The NF-κB inhibitor (E)-3-(4-methyl- phenylsulfonyl)-2-propenenitrile (BAY11-7082) was obtained from Sigma-Aldrich (St Louis, MO). Cells were treated with 20 ng/ml TNF-α (Sigma, USA) in the presence or absence of 1 mM metformin (24).
Wound healing assay
A scratch was made carefully with a sterile 200-µl pipette tip when adherent PC3cells reached 80% confluence. Then, the cells were incubated for another 24 h. Finally, photomicrographs were examined with a phase contrast microscope (Nikon Eclipse TE2000-U).
Invasion activity assay with Matrigel
The migration ability of PC3 cells was evaluated using the modified Boyden chamber method. PC3 cells were plated onto Matrigel-coated 8 µm filters with 20 ng/mL TNF-α in the presence or absence of 1 mM metformin. Outer wells were set as a chemoattractant filling with 800 µL of RPMI-1640 containing 10% FBS. Forty-eight hours after plating, the invading cells were the cells on the undersurface of the filter which were fixed, stained and counted.
Immunofluorescence
After serum starved for 12 h, PC3 cells which were cultured on chamber slides were treated with 20 ng/mL TNF-α with or without 1 mM metformin for 72 h. After being washed three times with PBS, the cells were fixed with 4% paraformaldehyde for 20 min and then permeabilized with 0.3% Triton X-100 for 10 min. Then, the cells were blocked with goat serum for 2 h at room temperature and incubated with specific antibodies for NF-κB-P65 (1:100 dilution, Cell Signaling Technology, USA) and E-cadherin (1:50 dilution, Cell Signaling Technology Cat# 14472, RRID: AB_2728770) at 4 °C overnight. After being washed three times with PBS, slides were incubated with FITC-conjugated secondary antibodies (1:100 dilution) for 2 h at room temperature. Nuclei were stained with DAPI for 5 min. Photomicrographs were captured with a microscope (OLYMPUS) to analyze the expression of NF-κB-P65 and E-cadherin (400×).
Western blot analyses
Western blotting was used to examine the protein expression levels of E-cadherin, N-cadherin, Vimentin, NF-κB-P65, p-IKK and p-IκBα. Briefly, proteins were extracted from PC3 cells, loaded onto 8–12% SDS-PAGE gels (30–50 µg/lane) and then transferred to nitrocellulose (Bio-Rad, Hercules, CA) filters. The primary antibodies used here were monoclonal mouse antibody against E-cadherin (1:1,000 dilution; Cell Signaling Technology Cat# 14472, RRID: AB_2728770) and polyclonal rabbit antibodies against N-cadherin (1:1,000 dilution; Cell Signaling Technology Cat# 4061, RRID: AB_10694647), Vimentin (1:1,000 dilution; Cell Signaling Technology Cat# 3295, RRID: AB_2216129), NF-κB-P65 (1:1,000 dilution; Cell Signaling Technology, USA), p-IKK (1:800 dilution; Cell Signaling Technology, USA), and p-IκBα (1:800 dilution; Cell Signaling Technology, USA).
Statistical analysis
All data are expressed as mean ± SEM. P values <0.05 were considered to indicate statistically significant differences. The means of the different groups were performed using Student’s t test.
Results
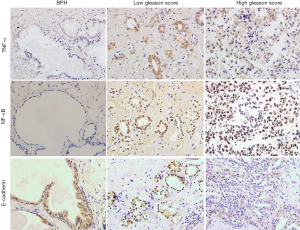
Expression of TNF-α and NF-κB-P65 was upregulated, while E-cadherin expression was downregulated in PCa specimens with a high Gleason score
As shown in Figure 1, TNF-α expression and NF-κB-P65 expression were mainly localized in the cytoplasm and nuclei of luminal cells of prostate carcinoma glands, while E-cadherin protein was localized in the cell membrane and cytoplasm of luminal cells of normal prostate glands and of prostate carcinoma cells. The expression of TNF-α and NF-κB-P65 was significantly increased in PCa cases, showing a highest expression in PCa cases with the high Gleason score. However, E-cadherin expression was significantly decreased in PCa cases, showing a lowest expression with the high Gleason score.
Metformin inhibited TNF-α-induced epithelial–mesenchymal transition in PC3 cells
First, the effects of metformin on TNF-α-induced EMT in PC3 prostate cancer cells were examined (Figure 2A,B). After being treated with TNF-α for 72 h, we found that the morphology of PC3 cells exhibited significant changes, and these PC3 cells exhibited more features of mesenchymal fibroblast-like and fusiform. Exposure of PC3 cells to TNF-α significantly reduced the expression of the epithelial marker E-cadherin (P<0.05) and induced the expression of the mesenchymal markers N-cadherin and Vimentin (P<0.05), which are changes characteristic of EMT. However, exposure of PC3 prostate cancer cells to TNF-α combined with metformin significantly restored the typical epithelial cobblestone morphology, restored the expression of the epithelial marker E-cadherin (P<0.05), and significantly downregulated the mesenchymal markers N-cadherin and Vimentin (P<0.05). Furthermore, as shown by immunofluorescence (Figure 2C), E-cadherin was mainly expressed in the cell membrane, and its expression was downregulated by TNF-α but upregulated after combined treatment with metformin.
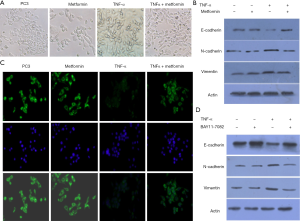
Then, the effects of BAY11-7082, an inhibitor of NF-κB, on the TNF-α-induced EMT process of PC3 prostate cancer cells were examined (Figure 2D). Consistent with the metformin results, BAY11-7082 also significantly restored the expression of the epithelial marker E-cadherin (P<0.05) and downregulated the expression of the mesenchymal markers N-cadherin and Vimentin (P<0.05).
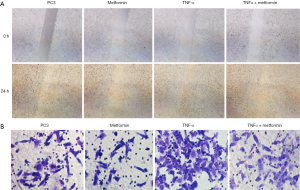
Metformin suppressed TNF-α-induced PC3 cell migration and invasion capacity
We examined the effect of TNF-α and metformin on prostate cancer cell migration using a wound healing assay and Boyden chamber invasion assay. As shown in Figure 3A,B, TNF-α promoted the migration and invasion ability of PC3 cells, but metformin significantly attenuated the migration and invasion ability of PC3 cells induced by TNF-α (P<0.05), consistent with our observation of the EMT pattern.
Metformin inactivated the NF-κB signaling pathway in PC3 cells
As shown in Figure 4A, exposure of PC3 cells to TNF-α upregulated the expression of p-IKK, p-IκBα, and NF-κB-P65. However, exposure of PC3 cells to TNF-α and metformin significantly downregulated the expression of p-IKK, p-IκBα, and NF-κB-P65 (P<0.05). Furthermore, as shown by immunofluorescence (Figure 4B), NF-κB-P65 was mainly expressed in the cytoplasm and nuclei of cells. Metformin downregulated the expression of NF-κB-P65 and inhibited its translocation into the nucleus.
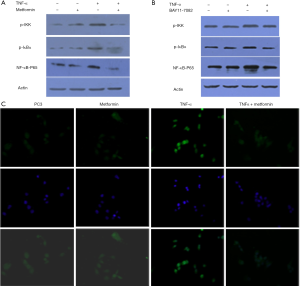
Moreover, the effects of BAY11-7082 on PC3 prostate cancer cells were examined (Figure 4C). Consistent with the metformin results, BAY11-7082 significantly down- regulated the expression of p-IKK, p-IκBα, and NF-κB-P65 (P<0.05).
Discussion
The present study demonstrated that the expression of TNF-α and NF-κB-P65 was significantly upregulated in PCa tissues and positively correlated with the Gleason score, while E-cadherin expression was significantly downregulated in PCa tissues and negatively correlated with the Gleason score. Moreover, metformin effectively inhibited the TNF-α-induced migration ability and invasion activity of PC3 cells. Furthermore, metformin might suppress the TNF-α-induced EMT process by inactivating the NF-κB signaling pathway.
Mortality in PCa patients is mainly caused by tumor metastasis. Recently, it was demonstrated that chronic inflammation plays a key role in PCa metastasis, which is a major challenge during PCa therapy. It is well known that inflammatory mediators, such as TNF-α, TGF-β and IL-6, are involved in the migration, invasion and metastasis of malignant cells (26). TNF-α is a major proinflammatory cytokine that participates in a series of biological activities, including inflammation, cell proliferation, cell differentiation and apoptosis (27). TNF-α is able to activate the canonical NF-κB pathway in various cell types. When cells are stimulated by TNF-α, IKK-β is first activated, and then the NF-κB inhibitor IκBα is phosphorylated and rapidly degraded. This allows the NF-κB heterodimer to translocate into the nucleus and activate the expression of numerous downstream target genes implicated in angiogenesis, the immune response, cell proliferation and cell apoptosis (28,29).
The EMT process can be induced by various growth factors and cytokines, which are produced by cell activation (30-32). We administered TNF-α to prostate cancer PC3 cells to investigate the effect of TNF-α on EMT characteristics in prostate cancer cells. Our results demonstrated that TNF-α-treated PC3 cells showed a significant increase in cell migration ability and invasion activity, suggesting a possible role of TNF-α in the migration behavior of prostate cancer cells. A hallmark of EMT is the loss of the cell adhesion molecule E-cadherin. Our results showed that TNF-α decreased the expression of E-cadherin and increased the expression of N-cadherin and Vimentin, but these changes were reversed by BAY11-7082, an inhibitor of NF-κB. These results demonstrated that TNF-α might promote the EMT process in prostate cancer cells via the NF-κB signaling pathway.
A series of in vitro and in vivo studies suggested that metformin might reduce the risk of biochemical recurrence and the rates of mortality in PCa (4,24). Although there have been kinds of researches on exploring the potential mechanism of metformin acting in PCa, the mechanism is not clear at all. This study showed that metformin alone had no effect on the NF-κB signaling pathway or EMT process. However, metformin combined with TNF-α successfully reduced the migration ability and invasion activity of prostate cancer cells induced by TNF-α. Moreover, metformin suppressed EMT, as demonstrated by the restored expression of E-cadherin and decreased the expression of N-cadherin and Vimentin.
TNF-α induces a series of inflammatory responses that further activate the NF-kB signaling pathway. Interestingly, this proinflammatory feedback loop also participates in prostate cancer. TNF-α significantly upregulated the expression of p-IKK, p-IκBα, and NF-κB-P65. However, metformin significantly lowered the TNF-α-induced expression of p-IKK, p-IκBα, and NF-κB-P65. Furthermore, metformin also inhibited NF-κB-P65 translocation into the nucleus. This study found that TNF-α promoted the EMT process, which was inhibited by BAY11-7082, an inhibitor of NF-κB. Consistent with our immunohistochemistry results in human prostate cancer tissues, TNF-α and NF-κB-P65 were positively correlated with the Gleason score, while E-cadherin expression was negatively correlated with the Gleason score. Therefore, we speculated that activation of the NF-κB signaling pathway might be involved in the EMT process in prostate cancer and prostate cancer progression and metastasis. Metformin might suppress the EMT process in prostate cancer and inactivate the TNF-α-induced NF-κB signaling pathway.
In conclusion, upregulation of TNF-α expression, activation of the NF-κB signaling pathway, and induction of the EMT process were involved in prostate cancer progression. In addition, metformin could suppress TNF-α-induced EMT in prostate cancer, potentially by inactivating the NF-κB signaling pathway.
Acknowledgments
We were grateful to Staffs in Key laboratory of Hubei Province for Digestive System Disease for their kind assistance.
Funding: This study was supported by
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1186
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1186). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (Wuhan, China) (No.: RM2018Y018-06) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol 2012;29:1314-27. [Crossref] [PubMed]
- Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008;27:3576-86. [Crossref] [PubMed]
- Akinyeke T, Matsumura S, Wang X, et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis 2013;34:2823-32. [Crossref] [PubMed]
- Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745-52. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev 2009;28:335-44. [Crossref] [PubMed]
- Yin T, Wang C, Liu T, et al. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J Surg Res 2007;141:196-203. [Crossref] [PubMed]
- Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007;39:305-18. [Crossref] [PubMed]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9:265-73. [Crossref] [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [Crossref] [PubMed]
- Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 2010;101:293-9. [Crossref] [PubMed]
- Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 2009;1796:75-90. [PubMed]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9:361-71. [Crossref] [PubMed]
- Wang H, Fang R, Wang XF, et al. Stabilization of Snail through AKT/GSK-3beta signaling pathway is required for TNF-alpha-induced epithelial-mesenchymal transition in prostate cancer PC3 cells. Eur J Pharmacol 2013;714:48-55. [Crossref] [PubMed]
- Ho MY, Tang SJ, Chuang MJ, et al. TNF-alpha induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3beta-dependent mechanism. Mol Cancer Res 2012;10:1109-19. [Crossref] [PubMed]
- Li CW, Xia W, Huo L, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res 2012;72:1290-300. [Crossref] [PubMed]
- Wang H, Wang HS, Zhou BH, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS One 2013;8:e56664. [Crossref] [PubMed]
- Kumar M, Allison DF, Baranova NN, et al. NF-kappaB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS One 2013;8:e68597. [Crossref] [PubMed]
- Techasen A, Namwat N, Loilome W, et al. Tumor necrosis factor-alpha (TNF-alpha) stimulates the epithelial-mesenchymal transition regulator Snail in cholangiocarcinoma. Med Oncol 2012;29:3083-91. [Crossref] [PubMed]
- Spratt DE, Zhang C, Zumsteg ZS, et al. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013;63:709-16. [Crossref] [PubMed]
- Rothermundt C, Hayoz S, Templeton AJ, et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09). Eur Urol 2014;66:468-74. [Crossref] [PubMed]
- Zhao Z, Cheng X, Wang Y, et al. Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS One 2014;9:e95884. [Crossref] [PubMed]
- Zhang J, Shen C, Wang L, et al. Metformin inhibits epithelial-mesenchymal transition in prostate cancer cells: involvement of the tumor suppressor miR30a and its target gene SOX4. Biochem Biophys Res Commun 2014;452:746-52. [Crossref] [PubMed]
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987;8:138-40. [PubMed]
- Aggarwal BB, Shishodia S, Sandur SK, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol 2006;72:1605-21. [Crossref] [PubMed]
- Choo MK, Sakurai H, Koizumi K, et al. Stimulation of cultured colon 26 cells with TNF-alpha promotes lung metastasis through the extracellular signal-regulated kinase pathway. Cancer Lett 2005;230:47-56. [Crossref] [PubMed]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749-59. [Crossref] [PubMed]
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest 2005;115:2625-32. [Crossref] [PubMed]
- Fan JM, Ng YY, Hill PA, et al. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 1999;56:1455-67. [Crossref] [PubMed]
- Morali OG, Delmas V, Moore R, et al. IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene 2001;20:4942-50. [Crossref] [PubMed]
- Strutz F, Zeisberg M, Ziyadeh FN, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 2002;61:1714-28. [Crossref] [PubMed]


