
Development of cancer diagnostics—from biomarkers to clinical tests
Introduction
The advent of molecular technologies has revealed a wealth of information about signaling pathways and gene regulation in cancer. New biomarkers and methods for classification of cancer subtypes, diagnosis, prognosis and prediction of response to therapy have been emerging. Advancements in analytical methods in molecular biology, such as polymerase chain reaction (PCR), deoxyribonucleic acid (DNA) arrays and next-generation sequencing have allowed researchers to interrogate a vast type of biological and clinical materials such as formalin-fixed, paraffin-embedded (FFPE) tissue, biopsies and cells present in blood, bone marrow or urine (1-8). Insights gained from the role and significance of the biomarkers in tumor tissues and cells will aid in understanding tumorigenesis and metastasis processes. In addition, the recent finding that circulating tumor cells (CTCs) and circulating DNA in blood can also have diagnostic value in metastatic cancers allowing clinicians to use them as surrogate endpoints (9,10). Diagnostic tests based on such information should enable “real time” biopsies of cancer progression and response to therapy. These new molecular and cellular technologies will enable more precise and objective decision-making.
On the other hand, many of the techniques that are employed today by pathologists and oncologists to generate a diagnosis, prognosis or prediction of therapy response have not changed over several decades. The fact highlights the challenges faced by new molecular and cellular technologies in having a real impact on patient management in clinic. One of the key challenges is to demonstrate the clinical value of a diagnostic test. For example, in the area of susceptibility/risk assessment, companies have commercialized molecular tests on the BRCA1 and BRCA2 genes for breast cancer (11). In the area of prognosis and prediction for therapy response, reverse transcription polymerase chain reaction (RT-PCR) based Oncotype Dx assay have also been adopted for breast cancer in predicting patients’ benefit with chemotherapy (12). In addition, in situ hybridization (ISH) assays based human epidermal growth factor receptor 2 (Her-2) test and anaplastic lymphoma kinase (ALK) test have been used to predict responses to targeted therapies such as Herceptin and Xalkori in breast cancer and lung cancer, respectively (13,14). In addition to clinical value, a routine test in clinic needs to be optimized so that the assay can fit into the clinical laboratory workflow and the assay result can be generated timely and reproducibly.
The review will focus on development of molecular and cellular diagnostic assays that have the potential to aid clinical decision-making and patient management in oncology. The process described here demonstrates the steps to translate and develop novel biomarkers into quality diagnostic tests that can be readily deployed into clinical laboratories. The examples referenced here illustrate how tissue- and cancer-specific biomarkers, coupled with new molecular technologies, can add value to conventional diagnostic methods by providing standardized, objective and highly informative diagnostic tests. These new tests will impact not only the business of diagnostics from a low margin, single measurement science to a high value, information intensive science, but also, with acceptance by clinicians, the way medicine is practiced in the future.
Assay development process
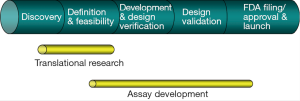
Thousands of papers published every year reveal new genes as potential biomarkers for cancer diagnosis. However, a few of these biomarkers are really used as cancer diagnostics in clinic. Figure 1 highlights the process to establish a specific biomarker as a diagnostic assay, which will require discovery of biomarkers, translational research, develop the biomarkers into diagnostic assays, incorporation of the assays in clinical trials to correlate the biomarkers with therapeutic responses and patient outcomes (Figure 1). One need to go through the entire development of a diagnostic assay with the new marker(s), which ensures that the assay used in the clinical trials are highly robust and reproducible to detect the intended disease state.
Biomarker discovery
Studies have shown that a wide variety of genomic changes, such as amplifications, translocations, deletions and point mutations may be present in a given type of cancer. Analysis of these genomic alterations led to the identification of oncogenes and tumor-suppressor genes involved in cancer development. Cancer development on the other hand is not restricted to genetic alterations. It can also be traced to epigenetic changes and changes in gene and protein expression levels. Studies of alterations in genetic, epigenetic and expression processes can help establish diagnostic biomarkers of tumors and classification of tumors based on recognition of complex molecular profiles or unique molecular alterations that occur in specific tumor types. However, it’s very difficult to achieve such objectives in practice for several reasons: the cross talk of different cancer-related pathways complicates the understanding of cancer biology; there is considerable heterogeneity in the tumor and functions of the genes among individuals with same types of cancers; the treatment targets are not absolutely specific to cancer cells; the effectiveness of the treatments is limited because the targets are affected by other factors and the functions of the targets may change over time and produce resistance to the treatment.
Cancer diagnosis is mainly carried out by examining morphology and antibody staining in biopsy or resected tissue samples. The recent development of diagnostic methods based on analyses of CTC and circulating DNA (ctDNA) in blood opened new avenues for cancer detection and prognosis (9,10). CTCs and ctDNA from cancer patients are now analyzed to detect tumor markers such as mutations, microsatellite instability, hypermethylation and gene expression. It is also possible to detect cancer cells from other body fluids such as saliva, urine, broncho alveolar lavage, sputum and ductal lavage because epithelial tumors grow and cancer cells can be sloughed off the tumors into body fluids. This makes it possible to detect molecular markers using these samples.
In situations where not much is known about a particular disease state, there is a need for discovery of biomarkers that determine the cause(s) of the disease or the genetic basis of susceptibility of the disease. Examples of high-throughput molecular discovery tools include genomics and next-generation sequencing. Clearly this discovery step of biomarkers is required for developing diagnostic assays, but there is sometimes a tendency to jump to the conclusion that the technology used for biomarker discovery can automatically be used as a diagnostic tool in clinic. It is worth noting that when considering the use of the biomarker and the technology platform in a clinical laboratory, additional development needs to be carried out in order to meet specific requirements in clinical practice, including facility and infrastructure requirements, labor and ancillary laboratory equipment needs and the cost structure. These can, in theory, all be overcome, but this is a reality that most biomarkers and discovery technologies can’t be directly used as diagnostics. The question remains as to whether or not the biomarker and the technology can become a clinically and economically feasible clinical tool. To answer the question requires a time- and resource-intensive development process.
Sample preparation
Sample preparation is a key pre-analytical step in diagnostic assay development. It ensures that the appropriate type of specimen is collected with a standard method and the handled in order to preserve specimen integrity. As to development of molecular assays, once the sample is obtained, it is important to confirm that DNA, RNA and proteins of the sample is stable and the specimen integrity is maintained during collection and transport. Numerous factors such as storage and transport time can affect quality of the specimen. Poorly handled samples may produce false negative test results. It’s necessary to verify the sample collection and handling conditions. Similarly, the handling and storage conditions of test reagents must be tracked and monitored to ensure that their composition, concentration, and function are well maintained. Other important factors include standard documentation, ensuing that personnel have been adequately trained and that laboratory equipment is correctly calibrated and functions properly.
Biospecimen repositories and biobanks will play an increasingly important role in development of diagnostic assays. The integrity of the samples and the availability of associated clinical data are vital to analytical verification and clinical validation of the diagnostic assay. In some cases, prospective studies will need to be undertaken. Throughout diagnostic assay development, access to patient specimens and detailed clinical data is a key requirement for all the stages of the development process. In certain cases such as prognostic assay development, patient outcome data will be required, dictating either that prospective trials be conducted or that retrospective studies on archived material be performed. The latter choice is attractive because commercialization of new assays can be accomplished sooner. There are millions of specimens in biobanks throughout the USA managed by clinical trials cooperative groups, academic institutions and individual investigators (15), and the National Cancer Institute has been working to unify these biobanks through a National Biospecimen Network (NBN). Other repositories also exist. For example, the Breast Cancer Family Registry has enrolled nearly 12,000 families containing individuals with a wide range of familial risks of breast cancer (16). It is an excellent source of tissue and data for studies that require large numbers of samples with epidemiological, clinical and molecular data. On the other hand, one should note that using banked samples collected from different clinical institutes has its own set of risks. First, a lack of standardization in tissue acquisition and annotation across laboratories should be dealt with. Secondly, the integrity of samples and isolated nucleic acids may vary widely across sites, depending on age of sample, fixation method, storage method, and so on. Lastly, the clinical data must be available, properly annotated and sorted through very carefully to ensure its proper association with the corresponding sample. Despite these limitations, archived samples remain a rich source of tissue and clinical data and will become a fixture of diagnostic assay development in the molecular medicine era.
Analytical assay development
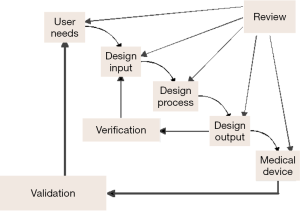
Analytical verification and clinical validation of candidate biomarkers from discovery involve the identification of contributing factors that affect test accuracy, reproducibility and interpretation (Figure 2). Figure 2 illustrates the definitions of each step in diagnostic assay development, especially verification and validation under design control. In developing a diagnostic test, one should recognize that the amount of process control required depends on the nature of the assay, the degree to which test reagents have been validated, the technology platform used for testing, and the assurance that specific regulatory approvals have been addressed, and that evolving knowledge regarding assay utility is incorporated into practice. For analytical and clinical assays to be considered reliable, it also requires statistically verifiable and reproducible results and a quality assurance process capable of aligning the various process elements with emerging knowledge. Accurate detection and quantification of the biomarker requires an assay capable of generating a reproducible result between the amount of input target in the sample and the output signal of the assay. Design and development of reproducible assays depend on the use of standards, reference materials and calibrators that contain known amount of the target. Samples containing concentration below the limit of detection of the assay will yield signals similar to the background noise of the assay. As the amount of input target in the sample is increased, a liner or near-linear signal response occurs over the assay’s dynamic range or range of quantification. The analytical lower limit of quantification is defined by the concentration at which the target can be detected with acceptable precision and accuracy. When the upper limit of quantification is reached, the signal saturates. Most conventional assays have a dynamic range of about or less than 3-4 logs (e.g., ELISA assays). Molecular assays may have dynamic ranges of 5-7 logs or more. Many analytical factors are important for relating the input concentration to the output signal of an assay. For example, the analytical accuracy of the assay assesses the degree of agreement between the measured target and the true value of that target. Analytical accuracy is typically assessed through comparison of a new method to an established test or a different type of test. Precision refers to the agreement of independent test results under defined conditions. Intra-assay precision refers to test reproducibility in the same analytical run. Inter-assay precision concerns test reproducibility among different runs. Precision among days, sites, lots and batches can also be assessed. Protocol standardization and documentation are required to facilitate the comparison, validation and integration of the assay and avoid variation of the test results from different laboratories.
Data interpretation
How test results are interpreted and acted upon represents an integral part of diagnostic assay development. The more accurate information conveyed to the doctor regarding the analytical and clinical performance of a test, the more likely that the appropriate clinical decisions will be made. Unfortunately many publications do not clearly describe or articulate the difference between analytical and clinical sensitivity and specificity. As the result, the implications of positive or negative test results for diagnosis and management are not sometimes clearly conveyed. For example, the analytical sensitivity is the smallest amount of target that can be reproducibly detected by a test. This is distinctly different from clinical or diagnostic sensitivity that is generally considered to reflect the ability of a test to correctly identify individuals who have an illness or specified clinical disorder. Analytical specificity is the ability of a test to accurately distinguish the target of interest from other substances in the sample. The clinical or diagnostic specificity is the ability of a test to identify people who do not have the illness or specified clinical disorder.
Quality assurance of the assay reports can also be challenging. If multiple analytical tests are used to assess the clinical status of patients, clinicians may not be aware of the appropriate tests to request or be knowledgeable about the precise interpretation of the results. Another reality is that clinicians often deal with multiple laboratories, each of which may be involved in performing different tests. As a result, no comprehensive summary of the test results may be available. Individuals may also be seen by multiple clinicians who may not have the results of previous tests. Similarly, a laboratory may not have access to a previous result that might help it ensure that the appropriate testing algorithm is performed. In the long term and especially when test complexity is high, it is likely that an electronic longitudinal patient record of test results would be an effective way of ensuring that test results are available to support best clinical practices.
Because of the rapid evolution of both diagnostics techniques and therapeutic interventions, a need clearly exists for greater cooperation between clinicians, laboratories, researchers, and regulatory authorities to better define the analytical and clinical performance characteristics of tests. Proper validation of complex assays with sufficient statistical rigor must be thought of as a requirement rather than an optional step in the commercialization of the assays (17-19). For example, the validation studies should be based on patient cohorts that are sufficiently homogeneous for the test to be developed. The patient cohort in the validation set should be independent of the training set. Both training and testing sets should be large enough to enable the investigator to employ either cross-validation or split sample validation. Regulatory agencies such as Food and Drug Administration (FDA) may have suggested criteria on sample size determination as well (CFR - Code of Federal Regulations Title 21). FDA classifies in vitro diagnostic device (IVD) products into Class I, II, or III according to the level of regulatory control that is necessary to assure safety and effectiveness. The classification of an IVD (or other medical device) determines the appropriate premarket process. Independent validation is a prerequisite for adoption into clinical practice. Such validation studies should employ a ‘locked’ version of the assay, algorithm and cutoffs and should be of sufficient size to permit determination of the accuracy of the assay result, with confidence intervals.
Examples of cancer diagnostic assays
Biopsy and diagnosis of carcinoma of unknown primary (CUP)
CUP refers to wherein metastatic disease is present without an identifiable primary tumor site. It represents approximately 3-5% of all cancers (20). The prognosis and therapeutic regimen of cancer patients are dependent on the origin of the primary tumor, underscoring the need to identify the site of the primary tumor.
A variety of methods are currently used to resolve this problem. Immunohistochemical (IHC) markers, using panels of 4-14 tissue specific markers to improve sensitivity and specificity and identify tumor of origin, have demonstrated accuracies of 66-88% (21). More expensive diagnostic workups include imaging methods, such as chest X-ray, computed tomographic (CT) scans, and positron emission tomographic (PET) scans. Despite these sophisticated technologies, the ability to resolve CUP cases is only 20-30% ante mortem.
A promising new approach lies in the ability of gene expression or microRNA profiling to identify the origin of tumors (22-25). The technologies are able to utilize FFPE tissue of the metastatic tumor, since fixed tissue samples are the standard material in current practice. qRT-PCR has been shown to generate reliable results from FFPE tissue but, from a practical point of view, requires a smaller set of tissue specific gene markers. The assays are currently provided as CAP/CLIA laboratory service.
Diagnosis of prostate cancer
Prostate cancer is the second leading cause of male cancer-related death in the US, and its prevalence increases with age. In men with elevated prostate-specific antigen (PSA) levels or abnormal digital rectal examination (DRE) findings, the standard for prostate cancer detection has been trans-rectal ultrasound-guided sextant needle biopsy, a method introduced in 1989 by Hodge (26). However, the sensitivity of biopsy may be suboptimal, especially for larger and eccentrically shaped prostates, with false-negative rates as high as about 20% (26).
Insights into the molecular pathogenesis of prostate cancer have identified new markers. For example, glutathione-S-transferase P1 (GSTP1) gene encodes the glutathione-S-transferase π enzyme, which is a member of a large family of glutathione transferases that function to protect cells from oxidative insult. GSTP1 has been extensively studied in prostate cancer, and its reduced expression, predominantly due to promoter hypermethylation, represents the most common epigenetic alteration associated with prostate cancer (27). Several studies have shown a high sensitivity for GSTP1 to detect the presence of both prostatic intraepithelial neoplasia and prostate cancer, an ability to distinguish these from benign prostatic hyperplasia (BPH), and a prevalence of methylation in the range of 70-90% in prostate cancer (28-30).
A second dilemma exists in prostate cancer screening. Currently, screening is accomplished using DRE and measurement of PSA levels in serum, which is sufficiently sensitive but not specific to render a diagnosis of cancer (31). Confirmatory diagnosis via a trans-rectal biopsy is required. Prostate cancer screening could benefit from a test that demonstrated a high specificity and that could be used in conjunction with PSA testing, in order to determine which patients should actually undergo a biopsy. In fact, the methylation detection of several molecular markers could also have clinical utility in the screening setting. In addition, a different assay proposed for use in this setting is based on detection of the mRNA for two genes, prostate cancer antigen 3 (PCA3) and PSA (31-33) (Table 1).

Full table
Prognosis of breast cancer
Breast cancer is a heterogeneous disease that exhibits a wide variety of clinical presentations, histological types and growth rates. As a result of these variations, determining prognosis for an individual patient at the time of initial diagnosis requires careful assessment of multiple clinical and pathological parameters; however, traditional prognostic factors are not always sufficient to predict patient outcomes accurately (34,35). In primary breast cancer, metastasis to axillary lymph nodes is the most important clinical prognostic factor. Approximately 60-70% of lymph-node-negative (LNN) patients are cured by local–regional treatment alone (35), while most patients who relapse will eventually die from their disease. Therefore, identification of those patients that are at high risk for relapse would enable a physician to prescribe adjuvant systemic therapy selectively to those patients without giving adjuvant therapy to all LNN patients.
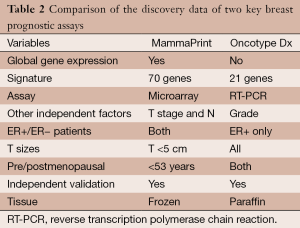
Genomic Health (CA, USA) has commercialized the Oncotype Dx assay, a set of 16 signature genes in their RNA expression and five control genes (11,36,37). As the signature was developed using data from patients who were treated with tamoxifen, it is not purely prognostic and is valid for estrogen-receptor-positive (ER+) patients initially and subsequently validated in other subtypes of breast cancer. Agendia (Amsterdam, The Netherlands) is commercializing another product for use in this setting referred to as the Mammaprint assay. It is a 70-gene signature based on the early work by Van’t Veer and colleagues (38-41). The signature is valid for women under the age of 55. These two assays have been offered as reference laboratory services commercially (Table 2). Other assays in this area use a 76-gene signature or a ratio of the expression of two genes and is proposed to predict recurrence in patients treated with adjuvant tamoxifen (42,43).
Full table
Circulating tumor cells (CTCs)
CTCs are rare, occurring at a frequency of one tumor cell for every million peripheral blood mononuclear cells (10). The number of patients exhibiting CTCs, and their absolute numbers of CTCs per patient increase as clinical stage rises (38). A 10,000-fold enrichment of CTCs in blood can be achieved by the use of ferrofluids linked to antibodies to the transmembrane glycoprotein epithelial cellular adhesion molecule (EpCAM) (44-47). For example, CellSearch could detect, enumerate and characterize CTCs, defined as nucleic acid-positive/CD45-negative/cytokeratin-positive, in the blood. Using the technology platform, studies can be designed to assess the clinical significance of CTCs in metastatic breast, colorectal and prostate cancer. Allard and colleagues (48) have demonstrated that the enumeration is linear over two logs (5-1,142 cells), that only one of 344 (0.3%) of healthy subjects had two or more CTCs per 7.5 mL of blood, and that, in 2,183 blood samples from 964 metastatic carcinoma patients, CTCs ranged from 0 to 23,618 per 7.5 mL of blood, with 36% exhibiting two or more CTCs. Of the major cancers, a larger percentage of prostate (57%) and breast (37%) cancer patients exhibited two or more CTCs. In a prospective, multicenter study, 177 patients with metastatic breast cancer were tested for levels of CTCs before treatment and at the first follow-up visit (43,49,50). Multivariate Cox proportional-hazards regression demonstrated that the levels of CTCs at baseline and at the first follow-up visit were the most significant prognostic factor of progression-free survival (PFS) and overall survival (OS).
Circulating endothelial cells (CECs) have also generated interest as a surrogate marker as CEC levels correlate with disease progression and reflect changes in the VEGF pathway (51,52). Angiogenesis plays an essential role in the growth and metastasis of tumors (51). Therefore, various anti-angiogenic agents are under development, targeting the vascular endothelial growth factor (VEGF) pathway (51). Reduction in the number of CECs accompanied a reduction in peripheral blasts in patients with refractory hematological malignancies who were treated with a microtubule inhibitor (53,54).
Enrichment of circulating cells can also enable a number of downstream applications. O’Hara and colleagues coupled in vitro transcription with multigene RT-PCR to analyze expression of 37 genes in CTCs (55). Smirnov and colleagues have amplified the RNA extracted from the CTC-enriched and CTC-depleted portions and applied this material to DNA arrays and have analyzed RNA extracted from enriched CTCs using qRT-PCR (56). Fehm and colleagues performed fluorescence in situ hybridization on CECs and demonstrated that patients had CECs that showed abnormal copy numbers (57).
Future directions
Novel diagnostics offers high sensitivity and high specificity in detection of cancer disease. In addition to the high sensitivity and specificity, these assays become accepted in clinical diagnostics owing to the ease with which they can be configured to detect almost any target, their requirement for minimal quantities of sample and their ability to be automated. Recent advances also allow such assays to be configured in a multiplex format, enabling simultaneous detection of multiple markers, which can be used to facilitate treatment of the disease. In addition, molecular markers of disease are stable, and the same assay chemistries can be used to develop diagnostic tests regardless of the type of disease being tested for.
There is a vast array of new technologies available, and they all have specific trade-offs with respect to speed, ease of use, throughput, multiplex level, ability to quantify, cost, availability of platform and resolution. It is important to determine the particular application for the test and specificity and sensitivity required for the application. Careful evaluation of specific needs will allow assay developers to choose solutions that are optimal for their specific needs. Failure to consider the strengths and weaknesses of each option will result in unnecessary costs and may limit effectiveness.
The discovery, validation, commercialization and clinical adoption of novel cancer diagnostic assays will change the paradigm of medical practice from single measurement, pathology- and clinical exam-driven decisions to more of an integrative approach in cancer patient management. Combining new medical content with emerging technologies and informatics will enable personalized medicine to reach its full potential. However, before these new technologies can reach the clinicians, issues in marker validation, sample acquisition and assay and platform development will have to be addressed. The focus of effort will have to shift from purely biomarker discovery to a more comprehensive approach that combines marker discovery, translational research, assay development and clinical validation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jian-Bing Fan) for the series “Application of Genomic Technologies in Cancer Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: An employee of the Roche Group.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Milani L. Syvä nen AC. Genotyping single nucleotide polymorphisms by multiplex minisequencing using tag-arrays. Methods Mol Biol 2009;529:215-29. [PubMed]
- Krjutskov K, Andreson R, Mägi R, et al. Development of a single tube 640-plex genotyping method for detection of nucleic acid variations on microarrays. Nucleic Acids Res 2008;36:e75 [PubMed]
- Tsouma A, Aggeli C, Lembessis P, et al. Multiplex RT-PCR-based detections of CEA, CK20 and EGFR in colorectal cancer patients. World J Gastroenterol 2010;16:5965-74. [PubMed]
- Brown PO, Botstein D. Exploring the new world of the genome with DNA microarrays. Nat Genet 1999;21:33-7. [PubMed]
- Ma CX, Ellis MJ. The Cancer Genome Atlas: clinical applications for breast cancer. Oncology (Williston Park) 2013;27:1263-9,1274-9.
- Ma S, Funk CC, Price ND. Systems approaches to molecular cancer diagnostics. Discov Med 2010;10:531-42. [PubMed]
- Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 2010;11:685-96. [PubMed]
- Ocaña A, Pandiella A. Personalized therapies in the cancer "omics" era. Mol Cancer 2010;9:202. [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [PubMed]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004;4:448-56. [PubMed]
- Shattuck-Eidens D, Oliphant A, McClure M, et al. BRCA1 sequence analysis in women at high risk for susceptibility mutations. Risk factor analysis and implications for genetic testing. JAMA 1997;278:1242-50. [PubMed]
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-26. [PubMed]
- Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA 2004;291:1972-7. [PubMed]
- Camidge DR, Theodoro M, Maxson DA, et al. Correlations between the percentage of tumor cells showing an anaplastic lymphoma kinase (ALK) gene rearrangement, ALK signal copy number, and response to crizotinib therapy in ALK fluorescence in situ hybridization-positive nonsmall cell lung cancer. Cancer 2012;118:4486-94. [PubMed]
- Hede K. NCI's National Biospecimen Network: too early or too late? J Natl Cancer Inst 2005;97:247-8. [PubMed]
- John EM, Hopper JL, Beck JC, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 2004;6:R375-89. [PubMed]
- Reid JF, Lusa L, De Cecco L, et al. Limits of predictive models using microarray data for breast cancer clinical treatment outcome. J Natl Cancer Inst 2005;97:927-30. [PubMed]
- Simon R. Development and validation of therapeutically relevant multi-gene biomarker classifiers. J Natl Cancer Inst 2005;97:866-7. [PubMed]
- Ransohoff DF. Rules of evidence for cancer molecular-marker discovery and validation. Nat Rev Cancer 2004;4:309-14. [PubMed]
- Ghosh L, Dahut W, Kakar S, et al. Management of patients with metastatic cancer of unknown primary. Curr Probl Surg 2005;42:12-66. [PubMed]
- Varadhachary GR, Abbruzzese JL, Lenzi R. Diagnostic strategies for unknown primary cancer. Cancer 2004;100:1776-85. [PubMed]
- Talantov D, Baden J, Jatkoe T, et al. A quantitative reverse transcriptase-polymerase chain reaction assay to identify metastatic carcinoma tissue of origin. J Mol Diagn 2006;8:320-9. [PubMed]
- Erlander MG, Ma XJ, Kesty NC, et al. Performance and clinical evaluation of the 92-gene real-time PCR assay for tumor classification. J Mol Diagn 2011;13:493-503. [PubMed]
- Pentheroudakis G, Pavlidis N, Fountzilas G, et al. Novel microRNA-based assay demonstrates 92% agreement with diagnosis based on clinicopathologic and management data in a cohort of patients with carcinoma of unknown primary. Mol Cancer 2013;12:57. [PubMed]
- Oien KA, Dennis JL. Diagnostic work-up of carcinoma of unknown primary: from immunohistochemistry to molecular profiling. Ann Oncol 2012;23:x271-7. [PubMed]
- Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol 1989;142:66-70. [PubMed]
- Baylin SB, Herman JG, Graff JR, et al. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998;72:141-96. [PubMed]
- Tokumaru Y, Harden SV, Sun DI, et al. Optimal use of a panel of methylation markers with GSTP1 hypermethylation in the diagnosis of prostate adenocarcinoma. Clin Cancer Res 2004;10:5518-22. [PubMed]
- Zhou M, Tokumaru Y, Sidransky D, et al. Quantitative GSTP1 methylation levels correlate with Gleason grade and tumor volume in prostate needle biopsies. J Urol 2004;171:2195-8. [PubMed]
- Eggener SE, Roehl KA, Catalona WJ. Predictors of subsequent prostate cancer in men with a prostate specific antigen of 2.6 to 4.0 ng/ml and an initially negative biopsy. J Urol 2005;174:500-4. [PubMed]
- Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA 2005;294:66-70. [PubMed]
- Birnbaum JK, Feng Z, Gulati R, et al. Projecting Benefits and Harms of Novel Cancer Screening Biomarkers: A Study of PCA3 and Prostate Cancer. Cancer Epidemiol Biomarkers Prev 2015;24:677-82. [PubMed]
- Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 2014;311:1143-9. [PubMed]
- Clahsen PC, van de Velde CJ, Goldhirsch A, et al. Overview of randomized perioperative polychemotherapy trials in women with early-stage breast cancer. J Clin Oncol 1997;15:2526-35. [PubMed]
- Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst 2001;93:979-89. [PubMed]
- Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem 2007;53:1084-91. [PubMed]
- Yang M, Rajan S, Issa AM. Cost effectiveness of gene expression profiling for early stage breast cancer: a decision-analytic model. Cancer 2012;118:5163-70. [PubMed]
- van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002;415:530-6. [PubMed]
- van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999-2009. [PubMed]
- Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190-7. [PubMed]
- Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics 2006;7:278. [PubMed]
- Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005;365:671-9. [PubMed]
- Ma XJ, Wang Z, Ryan PD, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 2004;5:607-16. [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [PubMed]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. [PubMed]
- Smerage JB, Hayes DF. The measurement and therapeutic implications of circulating tumour cells in breast cancer. Br J Cancer 2006;94:8-12. [PubMed]
- Racila E, Euhus D, Weiss AJ, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A 1998;95:4589-94. [PubMed]
- Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004;35:122-8. [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [PubMed]
- Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30. [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [PubMed]
- Mancuso P, Burlini A, Pruneri G, et al. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 2001;97:3658-61. [PubMed]
- Beerepoot LV, Mehra N, Vermaat JS, et al. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol 2004;15:139-45. [PubMed]
- Beaudry P, Force J, Naumov GN, et al. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res 2005;11:3514-22. [PubMed]
- O'Hara SM, Moreno JG, Zweitzig DR, et al. Multigene reverse transcription-PCR profiling of circulating tumor cells in hormone-refractory prostate cancer. Clin Chem 2004;50:826-35. [PubMed]
- Smirnov DA, Zweitzig DR, Foulk BW, et al. Global gene expression profiling of circulating tumor cells. Cancer Res 2005;65:4993-7. [PubMed]
- Fehm T, Sagalowsky A, Clifford E, et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res 2002;8:2073-84. [PubMed]


