
Polo-like kinase 1 is involved in apoptosis, invasion, and metastasis of pancreatic ductal adenocarcinoma
Introduction
According to statistics from America and Europe, the prognosis of pancreatic cancer (PC) is very poor, ranking it the fourth leading cause of cancer deaths (1). Pancreatectomy is the only effective method to cure this disease, but only 20% of patients are provided with the opportunity to resect the cancer tissue (2). Despite all these efforts, most of the patients are known to relapse (3). Distant metastasis is a major cause of death in cancer patients, especially with respect to PC. Only few cases do not show resistance to chemo- or radiation therapy (4). Patients with metastatic disease have a median survival of 6 months and a 5-year survival rate of 1%, whereas treatment with surgical resection and other therapeutic approaches is associated with 12–22 months of median survival and a 5-year survival rate of 20–25% (5). Hence, PC poses one of the greatest challenges in cancer research. Nevertheless, our increasing knowledge on the development of PC will help us find a cure for this debilitating disease (6-12). Nowadays, several signaling pathways were found as targets in PC, for examples, NF-κB is recognized as a key mediator of inflammation and has been frequently observed to play the crucial role in PC (13). Also, Hippo pathway, a key regulator of organ size, tissue hemostasis and regeneration, was reported to regulate the chemoresistance and prognosis in PC (14).
Polo-like kinase 1 (Plk1) is an important serine/threonine kinase involved in the process of mitosis. Plk1 is highly expressed in most neoplastic tissues compared to healthy tissues (15-18), and its deregulation affects the occurrence and development of tumors (19,20). It is reported that Plk1 could enhance efficacy of Olaparib in castration-resistant prostate cancer (21). Cervical cancer growth was also regulated via c-ABL-Plk1 axis (22). Our previous reports showed that down-regulating Plk1 can activate apoptosis-related pathways, such as caspase-related and Bcl-2 family-mediated pathways, leading to cell death. Furthermore, our findings confirm that suppression of PI3K/Akt and Plk1 combined with gemcitabine could be a potential therapeutic regimen for PC patients, but further efforts are needed (23).
A limited number of studies have demonstrated the possible relationship between the negative regulation of Plk1 and the development of tumors (24-28). Some cancers, like colorectal carcinoma and lung cancer, are caused by K-RAS mutations. Cells with high expression of K-RAS can be selectively killed without hurting normal cells by silencing Plk1 expression. In vitro and in vivo experiments have highlighted different ways to influence the activity and/or function of Plk1 for induction of tumor cell apoptosis (29,30). A lot of research is committed to using Plk1 as a means for targeted cancer treatment, especially through the development of small molecule inhibitors, which could be used as drugs for the treatment of various cancers (31-35). Knockdown of Plk1 using RNA interference technologies, like small interfering RNAs (siRNAs), led to cell cycle arrest and apoptosis of tumor cells, but had no effect on normal cells (36-38). Of note, 47.7% of invasive PCs are Plk1 positive, highlighting that Plk1 expression is closely related to malignancy of PC in some cases (39). In this study, we report that suppression of Plk1 expression in PC results in inhibition of cell invasion and migration, and induces apoptosis. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/tcr-20-1019).
Methods
Construction of recombinant adenovirus (rAd)-Plk1-shRNAs (rAd-shPlk1)
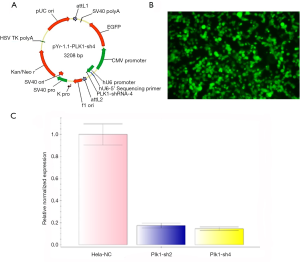
We selected different regions of the Plk1 transcript according to its gene sequence, and synthesized four shRNAs using the pYr-1.1 vector (hU6/EGFP/Neo) (Changsha Yingrun Biotechnology Co. Ltd, China) (Tables 1,2). After gene sequence analysis, pYr-1.1-Plk1-shRNA2 and pYr-1.1-Plk1-shRNA4 (Figure 1A) were introduced into the adenoviral vector pAd/PL-DEST (Changsha Yingrun Biotechnology Co. Ltd, China) through homologous recombination in vitro. HEK 293 cells were used for packing in to rAd (Figure 1B). By restructuring, both Plk1-sh2 and -sh4 could work effectively, and Plk1-sh4 proved superior to Plk1-sh2 (Figure 1C). Plk1-sh4 was finally selected for the following study, and was set as rAd-shPlk1, while rAd-EGFP (empty vector) served as experimental control.
Table 1
| Number | shRNA sequences (5'-3') |
|---|---|
| PLK1-shRNA1 | CGAGCTGCTTAATGACGAGTTCTCGAGAACTCGTCATTAAGCAGCTCGTTTTT |
| PLK1-shRNA2 | CCCGAGGTGCTGAGCAAGAAACTCGAGTTTCTTGCTCAGCACCTCGGGTTTTT |
| PLK1-shRNA3 | GCTCATCTTGTGCCCACTGATCTCGAGATCAGTGGGCACAAGATGAGCTTTTTT |
| PLK1-shRNA4 | GCAGCGTGCAGATCAACTTCTCTCGAGAGAAGTTGATCTGCACGCTGCTTTTTT |
Table 2
| Number | Target sequences (5'-3') | Target region |
|---|---|---|
| PLK1-shRNA1 | CGAGCTGCTTAATGACGAGTT | CDS |
| PLK1-shRNA2 | CCCGAGGTGCTGAGCAAGAAA | CDS |
| PLK1-shRNA3 | GCTCATCTTGTGCCCACTGAT | CDS |
| PLK1-shRNA4 | GCAGCGTGCAGATCAACTTCT | CDS |
Cell culture, adenovirus transduction, and RNAi
We selected the PC cell lines AsPC-1, BxPC-3, and PANC-1as research objects. The cells were cultured at 37 °C and 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 U/mL). They were divided into three groups: control group (untransduced cells), rAd-EGFP group (transduced with the empty vector control), and rAd-shPlk1 group (transduced with rAd-shPlk1). The cells were seeded on 6-well plates and cultured to 60% confluence prior to adenoviral transduction. For this, they were incubated with adenoviruses carrying vectors for expression of either EGFP or shPlk1 at a multiplicity of infection of approximately 106. Cells were collected for quantitative real-time PCR (qRT-PCR) and western blotting at 12 and 24 hours after transduction.
Detection of changes in Plk1 mRNA levels by qRT-PCR
TRIzol reagent was used for isolation of total RNA, and cDNA was synthesized according to the manufacturer’s instructions (Promega, Madison, WI, US). Afterwards, we employed the same amount of cDNA for each of the samples for analysis by qRT-PCR. Specific forward (5’-GCTGGGCAACCTTTTCCTG-3’) and reverse (5’-GCAGTGGGATCTGTCTGAAGC-3’) primers were used to detect Plk1gene expression in cells of the different groups. GAPDH served as internal standard, using the following primers: 5’-ATCCCATCACCATCTTCCAGG-3’ (forward) and 5’-CCATCACGCCACAGTTTCC-3’ (reverse). Plk1 and GAPDH gene amplifications were carried out for30 and 25 cycles, respectively.
Analysis of changes in Plk1 protein levels by western blotting
Cells were washed three times with phosphate buffered saline (PBS, 4 °C), and cellular protein was subsequently collected by lysis in 80 µL of RIPA buffer at 4 °C for 20 minutes. Removal of cell debris was performed by centrifugation at 12,000×g and 4 °C for 15 minutes, and the supernatant was collected for subsequent analyses. Afterwards, the Bio-Rad protein assay was used to determine the protein concentrations, and the same amount of protein (40 µg) per group was separated by electrophoresis, and subsequently transferred to polyvinylidene fluoride membranes (Amersham, USA). The membranes were blocked by incubation in 5% non-fat milk in Trisbuffered saline for 1 hour, followed by incubation with primary antibodies overnight at 4 °C. Anti-Plk1 monoclonal antibody (Cell Signaling Technology, Inc., USA) was used at 1 µg/mL. The membranes were then incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology, Inc., USA) at a 1:30,000 dilution. Electrochemiluminescence was detected on a ChemiDoc XRS+ Gel Imaging System. Next, in order to ensure that the same amount of protein had been loaded, the membranes were washed three times with Trisbuffered saline and re-incubated with an antibody directed against GAPDH.
Apoptosis analysis by flow cytometry
We collected the cells transduced with adenovirus at different time points, after which they were washed twice with PBS (4 °C), and re-dissolved in 1× binding buffer (BD Pharmingen, USA) to obtain a concentration of 1×106 cells/mL. Afterwards, 100 µL of the suspension (1×105 cells) were transferred to a 5 mL tube. After addition of 5 µL of APC AnnexinV (BD Pharmingen, USA) and 7-AAD (BD Pharmingen, USA) each, 400 µL of 1× binding buffer were added to each tube, after which the cells were incubated at 25 °C in the dark for 15 minutes. Cells were subsequently analyzed by flow cytometry (BD FACSCalibur equipped with CellQuest Pro) within 1 hour.
Transwell methods to detect cell migration and invasion
Sub-culturing of the cells in the upper chamber was performed until reaching 90% confluence. Next, 5×106 cells were suspended in 1 mL of serum-free culture medium. Half of the cell suspension (500 µL) was cultured in the upper chamber of the 24-welltranswell chamber, and 800 µL of culture medium mixed with 2% fetal bovine serum were added to the lower chamber. The Transwell cultures were cultured at 37 °C and 5% CO2 in an incubator for 12 hours to detect cell migration. Different from migration assays, matrix (Corning Matrigel) mixed with serum-free RPMI 1640 medium at a 1:5 ratio was added to the upper chamber, and the cells were then added and incubated at 37 °C and 5% CO2 for 24 hours before being used to detect cell invasion. The remaining liquid was removed before use. The cells were sub-cultured in the invasion chamber until reaching 80% confluence. As for the cell migration assay, 2.5×105 cells in 500 µL were cultured within one invasion chamber on a 24-well plate, and 800 µL culture medium mixed with 5% fetal bovine serum were added to the lower chamber. Subsequently, the cells were cultured under the aforementioned conditions for 24 hours to detect cell invasion. Following this, the cells on the upper surface of the membrane were cleared, and the cells on the underside of the membrane were stained with 0.1% crystal violet for 15 minutes. The samples were observed and imaged on a microscope at 10× to 20× total magnification, depending on the cell density. Crystal violet was rinsed off using 33% acetic acid, and the plate readout was performed on an ultraviolet spectrophotometer. Cell numbers were converted to OD values (except for AsPC-1 cells, which were counted at 100× magnification).
Statistical analysis
All of the experiments were repeated three to four times, and the data are presented as mean ± SD. Statistical analysis was performed using SPSS 21.0 software. Comparisons between two groups were conducted using Welch’s t-test. ANOVA was used for more than two groups, followed by Bonferroni test among different groups. Differences were considered statistically significant at P<0.05. P values indicating statistically significant differences were set as *P<0.05, **P<0.01, and ***P<0.001.
Results
Transduction with shPlk1 down-regulates Plk1 mRNA and protein levels in human PC cell lines
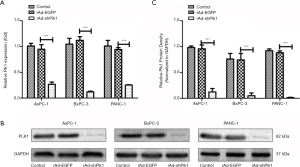
To detect the effect of shPlk1 on mRNA and protein levels of Plk1 in PC cell lines, we used qRT-PCR and western blotting, respectively. Results obtained by qRT-PCR indicated that the Plk1 mRNA levels were significantly different in the three groups, while total RNA levels were equal. More specifically, expression in the rAd-shPlk1 group was markedly lower than in the rAd-EGFP group (P=0.0006) (Figure 2A). Western blotting results indicated that the total amount ofPlk1 protein in the rAd-shPlk1 group was lower than in the rAd-EGFP group (P=0.0009) (Figure 2B,C). According to the above results, we found that Plk1 was down-regulated by shPlk1 with regard to both mRNA and protein levels in all PC cell lines tested.
Knockdown of Plk1 by shPlk1 induces apoptosis in PC cells
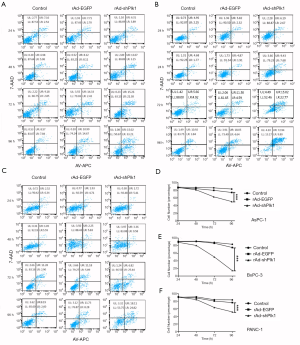
To test the effect of shPlk1-mediated down-regulation of Plk1 in pancreatic tumor cell lines, we used flow cytometry for analyzing apoptosis in the three groups. The results of this experiment are shown in Figure 3A,B,C. Cytograms of APC Annexin V binding (abscissa) versus 7-AAD uptake (ordinate) show three distinct populations: (I) viable cells (low APC and low 7-AAD signals) in gate LL; (II) early apoptotic cells (high APC and low 7-AAD signals) in gate LR, and (III) cells that have lost membrane integrity as a result of very late apoptosis (high APC and high 7-AAD signals) in gate UR. According to these data, we can conclude that within 24 hours, there is no significant difference in the three groups with regard to their apoptosis rates (P=0.058); however, after 48 hours, the apoptosis rate in the rAd-shPlk1 group was higher than in the other two groups (P=0.007). In line with this, the viable cell number in the rAd-shPlk1 group was significantly lower than in the rAd-EGFP group at all time points analyzed (P=0.003) (Figure 3D,E,F). Therefore, we inferred that shPlk1 could induce apoptosis of PC cells.
Down-regulation of Plk1 can inhibit PC cell invasion and migration
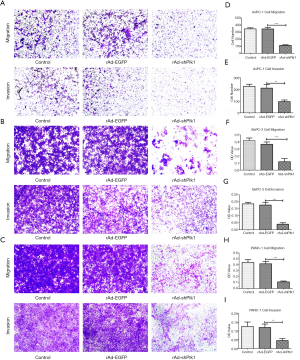
In order to detect whether down-regulation of Plk1 could affect PC cell invasion and migration, we transduced PC cells with rAd-shPlk1, and then evaluated cell migration and invasion capacities through transwell assays. Thereby, we showed that the number of cells that had migrated through the artificial basement membrane or/and Matrigel matrix from the rAd-shPlk1 group was significantly reduced compared the other two groups (P=0.004) (Figure 4).
Discussion
As mentioned above, the high mortality rate of PC patient urgently requires us to find the factors associated with cell proliferation, invasion, metastasis, and apoptosis, as well as the molecular mechanisms underlying these processes. Plk1, an indispensable factor in the process of cell proliferation, is highly expressed in a great proportion of PC cases, and in many other tumors (40). Lots of researches showed that metastasis in cancer patients and overall survival rates are associated with high Plk1 expression (41-43). Our previous study indicated that forced Plk1overexpression promoted cell proliferation and an increase in the percentage of cells in the G1/S phase, but a reduction in the G2/M phase cell population. We also found that siRNA-mediated knockdown of Plk1 caused cell cycle arrest in G2/M and proliferation inhibition (42). This research was devised to investigate the effect of Plk1 in apoptosis, invasion, and migration of human PC cell lines.
There are several strategies available, like anti-sense oligonucleotides (ASO), siRNAs, and small molecules, which can be employed to deplete or inhibit expression of Plk1 (43-46). However, in our present study, an shRNA against Plk1 was our method of choice on account of the same interference efficiency, but with a longer interference time compared to the siRNA-based strategy.
This was our first time to construct an adenoviral vector for introduction of shPlk1. In order to exclude that apoptosis induced by shPlk1 had an impact on invasion and migration, we examined PC cell apoptosis at different time points, and found that there was no significant difference in apoptosis induction among the three different conditions tested at 24 hours. Together, our data suggest that suppression of Plk1 expression caused an inhibition of invasion and migration capacities of PC cells. This finding powerfully supports the idea that Plk1 is a significant, decisive factor for invasion and metastasis of PC cells. Although our study showed that down-regulation of Plk1 exerts this effect on invasion and migration in PC cells, lower levels of Plk1 are not necessarily beneficial. The treatment effect of Plk1 in pancreatic cancer was still unknown. We need further study in vivo and vitro to verify the effect of Plk1 on pancreatic cancer. It was reported that down-regulating the expression of Plk1 could still lead to aneuploidy and tumorigenesis (47). Therefore, it is important to maintain endogenous levels of Plk1 for mitosis to proceed normally, and attention should be paid to side effects, such as tumorigenesis, should Plk1 inhibitors be used in clinical trials.
At present, it is believed that Plk1 leads to epithelial-mesenchymal transition and affects the invasion and metastasis of tumor tissues (48). Cai et al. found that Plk1 regulates both metastasis and epithelial-mesenchymal transition of gastric cancer cells through regulation of the AKT pathway, and Wu et al. showed Plk1 to be involve directly in the phosphorylation of CRAF, with subsequent stimulation of the MEK1/2-ERK1/2-Fra1-ZEB1/2 signaling pathway, thus leading to epithelial-mesenchymal transition, which in turn regulates invasion and metastasis of metastatic prostate cancer (49,50). During this study, the exact molecular mechanisms underlying the effects of Plk1on invasion and metastasis of PC were not investigated, and this will be addressed in future studies.
Conclusions
In conclusion, our study shows that the expression of Plk1 is closely related to invasion and metastasis of PC, and that shRNA-dependent down-regulation of Plk1 can lead to inhibition of PC cell migration and invasion in vitro. Further studies using animal models will pave the way for future clinical trials for the development and implementation of Plk1 inhibitors or RNAi-mediated Plk1 gene silencing strategies to complement current therapeutic approaches for PC. Certainly, further efforts to investigate the mechanisms behind PC development and progression induced by Plk1are warranted.
Acknowledgments
Funding: This work was supported in part by grants from
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-1019
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-1019
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-1019
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1019). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75-83; discussion 83. [Crossref] [PubMed]
- Long J, Zhang Y, Yu X, et al. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets 2011;15:817-28. [Crossref] [PubMed]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163-72. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231-7. [Crossref] [PubMed]
- Van Laethem JL, Verslype C, Iovanna JL, et al. New strategies and designs in pancreatic cancer research: consensus guidelines report from a European expert panel. Ann Oncol 2012;23:570-6. [Crossref] [PubMed]
- Matthaei H, Schulick RD, Hruban RH, et al. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol 2011;8:141-50. [Crossref] [PubMed]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [Crossref] [PubMed]
- Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010;467:1109-13. [Crossref] [PubMed]
- Garrido-Laguna I, Tan AC, Uson M, et al. Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br J Cancer 2010;103:649-55. [Crossref] [PubMed]
- Iacobuzio-Donahue CA. Genetic evolution of pancreatic cancer: lessons learnt from the pancreatic cancer genome sequencing project. Gut 2012;61:1085-94. [Crossref] [PubMed]
- Pramanik KC, Makena MR, Bhowmick K, et al. Advancement of NF-kappaB Signaling Pathway: A Novel Target in Pancreatic Cancer. Int J Mol Sci 2018;19:3890. [Crossref] [PubMed]
- Ansari D, Ohlsson H, Althini C, et al. The Hippo Signaling Pathway in Pancreatic Cancer. Anticancer Res 2019;39:3317-21. [Crossref] [PubMed]
- Weichert W, Denkert C, Schmidt M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer 2004;90:815-21. [Crossref] [PubMed]
- Weichert W, Schmidt M, Gekeler V, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate 2004;60:240-5. [Crossref] [PubMed]
- Takahashi T, Sano B, Nagata T, et al. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci 2003;94:148-52. [Crossref] [PubMed]
- Ito Y, Miyoshi E, Sasaki N, et al. Polo-like kinase 1 overexpression is an early event in the progression of papillary carcinoma. Br J Cancer 2004;90:414-8. [Crossref] [PubMed]
- Ando K, Ozaki T, Yamamoto H, et al. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem 2004;279:25549-61. [Crossref] [PubMed]
- Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer 2006;6:321-30. [Crossref] [PubMed]
- Li J, Wang R, Kong Y, et al. Targeting Plk1 to Enhance Efficacy of Olaparib in Castration-Resistant Prostate Cancer. Mol Cancer Ther 2017;16:469-79. [Crossref] [PubMed]
- Yang X, Chen G, Li W, et al. Cervical Cancer Growth Is Regulated by a c-ABL-PLK1 Signaling Axis. Cancer Res 2017;77:1142-54. [Crossref] [PubMed]
- Mao Y, Xi L, Li Q, et al. Combination of PI3K/Akt Pathway Inhibition and Plk1 Depletion Can Enhance Chemosensitivity to Gemcitabine in Pancreatic Carcinoma. Transl Oncol 2018;11:852-63. [Crossref] [PubMed]
- Schmit TL, Ledesma MC, Ahmad N. Modulating polo-like kinase 1 as a means for cancer chemoprevention. Pharm Res 2010;27:989-98. [Crossref] [PubMed]
- Benoit DS, Henry SM, Shubin AD, et al. pH-responsive polymeric sirna carriers sensitize multidrug resistant ovarian cancer cells to doxorubicin via knockdown of polo-like kinase 1. Mol Pharm 2010;7:442-55. [Crossref] [PubMed]
- Chun G, Bae D, Nickens K, et al. Polo-like kinase 1 enhances survival and mutagenesis after genotoxic stress in normal cells through cell cycle checkpoint bypass. Carcinogenesis 2010;31:785-93. [Crossref] [PubMed]
- Peter B, Gleixner KV, Cerny-Reiterer S, et al. Polo-like kinase-1 as a novel target in neoplastic mast cells: demonstration of growth-inhibitory effects of small interfering RNA and the Polo-like kinase-1 targeting drug BI 2536. Haematologica 2011;96:672-80. [Crossref] [PubMed]
- Kawata E, Ashihara E, Maekawa T. RNA interference against polo-like kinase-1 in advanced non-small cell lung cancers. J Clin Bioinforma 2011;1:6. [Crossref] [PubMed]
- Valianou M, Cox AM, Pichette B, et al. Pharmacological inhibition of Polo-like kinase 1 (PLK1) by BI-2536 decreases the viability and survival of hamartin and tuberin deficient cells via induction of apoptosis and attenuation of autophagy. Cell Cycle 2015;14:399-407. [Crossref] [PubMed]
- Weiss L, Efferth T. Polo-like kinase 1 as target for cancer therapy. Exp Hematol Oncol 2012;1:38. [Crossref] [PubMed]
- Mok WC, Wasser S, Tan T, et al. Polo-like kinase 1, a new therapeutic target in hepatocellular carcinoma. World J Gastroenterol 2012;18:3527-36. [Crossref] [PubMed]
- Berg T. Small-molecule inhibitors of protein-protein interactions. Curr Opin Drug Discov Devel 2008;11:666-74. [PubMed]
- McInnes C, Mezna M, Fischer PM. Progress in the discovery of polo-like kinase inhibitors. Curr Top Med Chem 2005;5:181-97. [Crossref] [PubMed]
- Schmit TL, Ahmad N. Regulation of mitosis via mitotic kinases: new opportunities for cancer management. Mol Cancer Ther 2007;6:1920-31. [Crossref] [PubMed]
- Schoffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 2009;14:559-70. [Crossref] [PubMed]
- Spankuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, et al. Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst 2002;94:1863-77. [Crossref] [PubMed]
- Jalili A, Moser A, Pashenkov M, et al. Polo-like kinase 1 is a potential therapeutic target in human melanoma. J Invest Dermatol 2011;131:1886-95. [Crossref] [PubMed]
- Nihal M, Stutz N, Schmit T, et al. Polo-like kinase 1 (Plk1) is expressed by cutaneous T-cell lymphomas (CTCLs), and its downregulation promotes cell cycle arrest and apoptosis. Cell Cycle 2011;10:1303-11. [Crossref] [PubMed]
- Weichert W, Schmidt M, Jacob J, et al. Overexpression of Polo-like kinase 1 is a common and early event in pancreatic cancer. Pancreatology 2005;5:259-65. [Crossref] [PubMed]
- Gray PJ Jr, Bearss DJ, Han H, et al. Identification of human polo-like kinase 1 as a potential therapeutic target in pancreatic cancer. Mol Cancer Ther 2004;3:641-6. [PubMed]
- Takai N, Hamanaka R, Yoshimatsu J, et al. Polo-like kinases (Plks) and cancer. Oncogene 2005;24:287-91. [Crossref] [PubMed]
- Kneisel L, Strebhardt K, Bernd A, et al. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol 2002;29:354-8. [Crossref] [PubMed]
- Qian Y, Hua E, Bisht K, et al. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis 2011;28:899-908. [Crossref] [PubMed]
- Yu C, Zhang X, Sun G, et al. RNA interference-mediated silencing of the polo-like kinase 1 gene enhances chemosensitivity to gemcitabine in pancreatic adenocarcinoma cells. J Cell Mol Med 2008;12:2334-49. [Crossref] [PubMed]
- Jimeno A, Rubio-Viqueira B, Rajeshkumar NV, et al. A fine-needle aspirate-based vulnerability assay identifies polo-like kinase 1 as a mediator of gemcitabine resistance in pancreatic cancer. Mol Cancer Ther 2010;9:311-8. [Crossref] [PubMed]
- Yao YD, Sun TM, Huang SY, et al. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci Transl Med 2012;4:130ra48. [Crossref] [PubMed]
- Lu LY, Yu X. The balance of Polo-like kinase 1 in tumorigenesis. Cell Div 2009;4:4. [Crossref] [PubMed]
- Fu Z, Wen D. The Emerging Role of Polo-Like Kinase 1 in Epithelial-Mesenchymal Transition and Tumor Metastasis. Cancers (Basel) 2017;9:131. [Crossref] [PubMed]
- Cai XP, Chen LD, Song HB, et al. PLK1 promotes epithelial-mesenchymal transition and metastasis of gastric carcinoma cells. Am J Transl Res 2016;8:4172-83. [PubMed]
- Wu J, Ivanov AI, Fisher PB, et al. Polo-like kinase 1 induces epithelial-to-mesenchymal transition and promotes epithelial cell motility by activating CRAF/ERK signaling. Elife 2016;5:e10734. [Crossref] [PubMed]


