
Proton therapy for gastrointestinal cancers
Introduction
Proton radiation therapy has gained popularity over the past few decades as a means of optimizing radiation treatment. In the field of radiation oncology there is an ever-present impetus to improve tumor killing while minimizing side effects. This is achieved by delivering higher doses of radiation to the tumor while sparing normal surrounding tissues. The most important factor in determining the success of this optimization, or therapeutic ratio, is tight control of dose conformality. However, the magnitude of normal tissue sparing in various regions of the body is variable due to specific individual anatomy. There is, in fact, evidence that patients with high-grade acute organ toxicity during multimodality treatment seem to benefit in regard to tumor response and prognosis (1-3). This places an even greater emphasis on the need to lower radiation exposure to organs at risk. There is still a paucity of data involving the use of proton therapy in gastrointestinal malignancy due to a relative lack of clinically available proton treatment facilities worldwide.
Most of the radiation given with conventional X-ray, or photon, therapy is deposited along the entrance and exit of the beam path. In contrast, protons are small charged particles that travel only a finite distance in tissue. Most of an accelerated proton’s energy is deposited as a Bragg peak at the end of the beam path. The depth of this Bragg peak can be modulated by either varying the proton beam energy or adding compensators to the treatment gantry. Therefore, the integral dose is greatly reduced since there is no exit dose and the entrance dose is greatly reduced relative to the Bragg peak. The ability to dose-escalate at the tumor while maintaining low toxicity in normal tissues may improve the therapeutic ratio of radiation treatment. The kidneys, for example, are often involved in the radiation fields when treating gastric or pancreatic cancer. There is evidence of decline in relative renal function following kidney irradiation (4). The degree of dysfunction correlates with the amount of radiation received. These adverse outcomes emphasize the importance of sparing normal tissue from dose during radiation therapy. The use of proton therapy for treating gastrointestinal (GI) malignancy is still a topic of many ongoing studies. Nonetheless, the opportunity to improve dose distribution to highly critical organs within the abdominal cavity presents itself as a major topic of interest. We present an overview of proton therapy contributions in the role of treating esophageal, gastric, pancreatic, and rectal malignancy.
Esophageal cancer
Esophageal cancer accounts for 5% of all GI cancers worldwide. It is the sixth leading cause of death from cancer worldwide. There is a male predominance, with the highest prevalence in Asia. The percentage and overall incidence of adenocarcinoma histology is increasing in comparison to squamous cell carcinoma histology. Tobacco, alcohol, gastroesophageal reflux (GERD), and Plummer-Vinson syndrome are known risk factors for esophageal cancer. Barrett’s esophagus is an established risk factor associated with a 9-fold risk of developing adenocarcinoma of the distal esophagus (5).
Treatment options are guided by disease stage. Early stage tumors with minimal invasion have very low risk of distant metastases. They are often treated with surgical resection of the tumor. Early stage tumors with deeper invasion are generally managed with esophagectomy. Concurrent chemotherapy and radiation may be considered in patients who are not surgical candidates. Locally advanced disease is managed with up-front concurrent chemoradiation followed by re-evaluation for possible esophagectomy. Concurrent chemoradiation is considered the standard of care, yielding increased survival benefit when compared to radiation alone (6). Meta-analyses have also demonstrated increased survival benefit when chemoradiation is administered pre-operatively as compared to pre-operative chemo alone or no pre-operative treatment (7). Furthermore, available data suggest improvement in local control and a possible survival improvement with the use of post-operative chemoradiation as well as post-operative radiation alone (8).
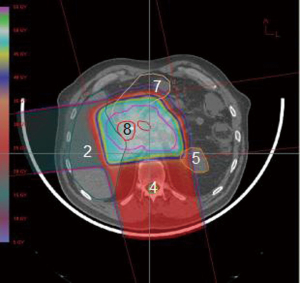
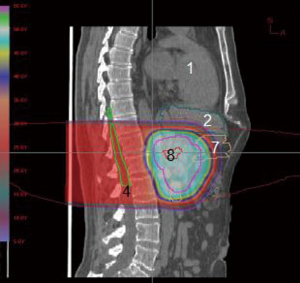
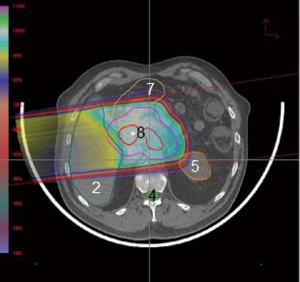
The esophagus is located in the posterior mediastinum in close proximity to several critical structures, namely lung, spinal cord, and heart. Minimizing toxicities to these critical structures decreases overall treatment morbidity and mortality to the patient. However, a margin large enough to cover the areas of tumor and involved lymph nodes must be accounted for in the radiation field. This puts surrounding organs at greater risk. Lung dose is a major risk factor for toxicity during irradiation for esophageal cancer. It is often necessary to use several beams oriented at oblique angles in order to keep spinal cord dose within tolerance (Figures 1,2,3,4). This results in a significant amount of radiation dose received by the lung, leading to subsequent radiation pneumonitis and post-operative pulmonary complications in some patients.
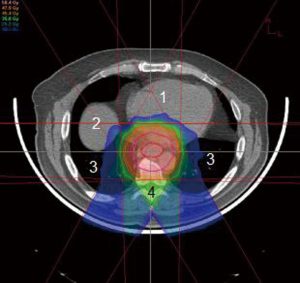
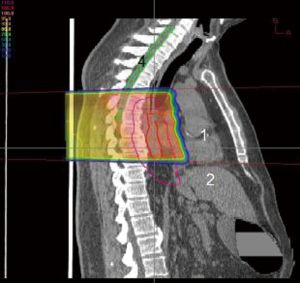
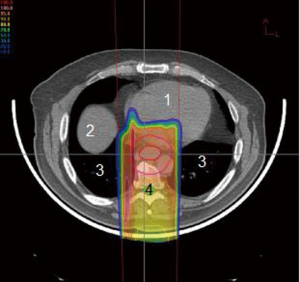
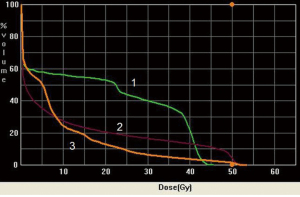
A recent phase III randomized prospective trial compared surgery alone with pre-operative concurrent chemotherapy using carboplatin and taxol given with 41.4 Gy conventional X-radiation (9). The standard radiation dose in most North American studies is 50.4 Gy, but despite the reduced dose regimen in this study the authors found improved median and overall survival in the pre-operatively treated arm compared to surgery alone. A dose of 41.4 Gy allowed the authors to use an anterior-posterior beam arrangement to spare integral dose in the lung while keeping the spinal cord dose within tolerance. The dosimetric properties of proton therapy could potentially allow safe dose escalation to 50.4 Gy or above while simultaneously sparing integral dose to the lungs and keeping the spinal cord dose within tolerance. A series of esophageal patients at M.D. Anderson Cancer Center treated with either IMRT or proton therapy found improved dose toxicity profiles when protons were used (10). While the dosimetric advantage of protons is clear, the reported clinical experience using proton beams is limited. Nonetheless several studies do report fewer interruptions during treatment due to radiation esophagitis and hematologic toxicities (11,12). The use of intensity-modulated proton beam therapy (IMPT) is the topic of several new trials in the management of esophageal cancer.
Gastric cancer
Gastric cancer has seen a sharp decrease in incidence in Western countries over the past 60 years. However, the incidence of gastro-esophageal and proximal gastric tumors is increasing. It is the third most common cancer in the world and the second leading cause of cancer deaths worldwide. There is a slight male predominance, with the median age of diagnosis at 65 years. The highest death rates from gastric cancer are reported in Asia and South America. Known risk factors are smoked and salted food, pernicious anemia, and Helicobacter Pylori infection. Adenocarcinoma comprises the vast majority of gastric cancer histology. Positron emission tomography (PET) has achieved an increasing role in the diagnosis and staging of gastric cancers and is used as an option for greater specificity in characterizing suspected gastric tumor (13). Anatomic imaging, however, remains the standard recommendation.
Surgery has been the mainstay of treatment, although chemotherapy and radiation now have an established role. Tumors of the upper and middle third of the stomach generally require a total gastrectomy, while partial gastrectomy may be adequate for tumors located in the distal antrum. These considerations are highly variable and specific to each patient. Achieving negative margins and thorough lymph node assessment is critical in gastric cancer treatment, as the majority of recurrences are locoregional (14).
Today, the standard of care for gastric cancer is tri-modality treatment or, in some institutions, perioperative chemotherapy. Surgery, chemotherapy, and radiation together all play an increasingly important role. Several landmark trials investigated the role of chemoradiation in relation to surgery. The INT0116 trial demonstrated an overall survival benefit (HR 1.32, P=0.0046) when surgery is followed by a combination of chemoradiation (15). Gastric cancer recurrence is largely locoregional in nature. Post-operative radiation therapy is generally given to the surgical bed and surrounding lymph node regions. This results in large radiation fields that put nearby organs at risk, including lungs, liver, kidney, and small intestine. Little or no clinical prospective data exist regarding proton therapy in gastric cancer. The inherent dosimetric advantage that proton therapy provides should serve as an opportunity for improving the post-gastrectomy bed normal-organ toxicities.
Pancreatic cancer
Despite being only the tenth most common cancer worldwide, pancreatic cancer is the fourth leading cause of cancer mortality. It is found primarily in Western countries. Known risk factors include tobacco use, ionizing radiation, and diets high in animal fat. The incidence has been stable over the past 20 years but has increased 3-fold since 1920. It is seen more frequently in African Americans and males, with a peak incidence at 70 to 80 years of age. The most common histologic cell type is adenocarcinoma, with mucinous, serous, and neuroendocrine histologies comprising less than 10% of cases. Although elevated in some benign conditions, the tumor marker CA 19-9 is often used as a pretreatment prognostic indicator. A decreasing value after pancreatic cancer treatment is associated with better survival (16).
As a whole, pancreatic cancer carries a very poor prognosis. Nearly 80% of newly diagnosed cases are stage IV disease. Its 5-year overall survival rate is among the lowest of all cancers. Over 80% of patients who undergo surgery will have recurrence. Historically, surgery with or without chemotherapy has been the mainstay of pancreatic cancer. Chemotherapy alone has not been shown to be curative in GI malignancies. However, some promising survival data are associated with the concurrent administration of intense cytotoxic chemotherapy regimens such as fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) (17). The role of novel molecularly targeted agents is a topic of active investigation as well. When pre-operative chemotherapy or chemoradiation is administered, it is critical to assess for disease response to this treatment. Patients with disease progression during pre-operative therapy likely will not benefit from surgery and an extremely morbid surgery may be prevented (18). Patients deemed resectable typically undergo pancreaticoduodenectomy, or Whipple’s Procedure, followed by chemoradiation. There is evidence for a survival benefit in giving post-operative chemoradiation over post-operative chemotherapy alone (19,20). Aside from extended survival, post-operative chemoradiation has been seen to improve performance status, reduce the amount of hospital stay, and facilitate greater pain relief (21). However, a consensus has not yet been reached defining the exact role of radiation in pancreatic cancer.
Since the value of radiation therapy in this disease has not been firmly established it is difficult to estimate the number of cases suitable for proton-beam therapy. Radiation dose escalation has shown disease control benefits for various cancer sites. Though systemic relapse is still a predominant feature, dose escalation has been shown to increase long-term disease control (22). Improvements in radiation treatment techniques, particularly in IMRT, have allowed dose escalation with acceptable normal tissue toxicitites (23). Few pancreatic proton dosimetric data are available, but one study did demonstrate the dosimetric feasibility of five fractions of 5 Gy delivered as pre-operative pancreatic cancer treatment (24)
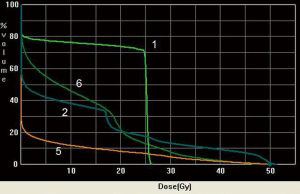
The pancreas is located in the retroperitoneum, closely abutting several critical organs. A proton beam’s unique qualities would seem to lend itself well to such a situation (Figures 5,6,7,8). One study compared target coverage and dose-volume histograms of proton therapy plans to various 3D conformal and IMRT photon plans (25). The proton therapy plans demonstrated significantly lower integral doses. In particular, the rapid downstream falloff of dose for tumors near the ligament of Treitz enabled complete coverage of the planning target volume while staying within acceptable normal-tissue toxicity limits. In Japan concurrent proton therapy with high-dose gemcitabine has been studied, showing high feasibility and tolerability (26). The frequency of grade 3 or higher acute GI toxicities was low even when using doses as high as 70.2 GyE. Major late toxicities varied, depending on pancreatic tumor position relative to organ anatomy. Nonetheless, they were significantly reduced when using a field-in-field technique, as in this study. Proton therapy will continue to be a major focal point of investigation in future pancreatic cancer dose-escalation studies.
Rectal cancer
The incidence of rectal cancer is equally distributed between males and females. The median age of diagnosis is the seventh decade. Associated risks factors include high-fat, low-fiber diets; animal fat; red meat; and inflammatory bowel disease. A number of gene mutations also are associated with a high risk of colon cancer. The colon and rectum are divided by the rectosigmoid junction at the level of the S3 vertebra. The rectum begins below this junction. In planning treatment for colorectal cancer one must take into account the highly variable lymph node drainage patterns, depending on the level of involvement in the colon or rectum.
The mainstay of treatment for rectal cancer remains surgery. Historically, however, surgery alone has yielded high cure rates only in early-stage rectal cancers. The addition of post-operative radiation improved local control rates but did not improve overall survival. When chemotherapy was combined with radiation, an improvement in local control, distant failures, and overall survival was seen (27). Unfortunately, many patients who undergo surgery are unable to complete chemoradiation. Therefore, interest grew in pre-operative chemoradiation therapy. The German rectal cancer study compared pre-operative with post-operative chemoradiation and found no difference in survival rates. Pre-operative chemoradiation, however, demonstrated improved local control rates and significantly improved toxicity rates (28). Many institutions now consider pre-operative chemoradiation to be the standard of care in rectal cancer.
Isacsson et al. initially demonstrated dosimetric advantages with proton therapy in inoperable rectal cancer patients (29). Three dose plans were made for each of six patients: one proton plan, one X-ray plan, and one mixed plan with X-ray beams followed by a proton beam boost. They demonstrated that the treatment plans involving proton beams showed superior dosimetric coverage of the target volumes. Wolff et al. performed a treatment planning comparison study for rectal cancer using various treatment modalities (30). Twenty-five patients with locally advanced rectal cancer were treated with pre-operative chemoradiation. The radiation was planned out using either Intensity modulated radiation therapy (IMRT), RapidArc with two arcs (full gantry rotation around the patient), 3D conformal therapy, and proton therapy. Consistently, improved systematic sparing of normal tissues as seen in the proton therapy plans while providing adequate coverage to the target regions.
Protons showed reliable and reproducible dosimetric advantages in these rectal cancer cases. The ability to spare nearby bladder, small bowel, and other normal tissue indicates an opportunity for an improved therapeutic ratio in locally advanced rectal cancers.
Anal cancer
The past three decades has seen a marked increase in the incidence of anal cancer. Overall it is still a relatively rare malignancy, comprising less than 2% of all gastrointestinal cancers. It is seen nearly twice as often in women than in men. The mean age of diagnosis is between ages 55 and 65. Human papilloma virus (HPV) infection is strongly associated with anal squamous cell carcinoma, which comprises over 75% of cases. It is thought that HPV infection, particularly HPV-16, 18 may in fact be a requisite for disease formation. Anal cancer is associated with AIDS, although, unlike cervical cancer, it is not an AIDS-defining illness. Other risk factors include cigarette smoking, multiple sexual partners, and a history of anal warts.
Historically, abdominoperineal resection (APR) was the standard treatment for anal cancer. This required a permanent colostomy. However, in 1973 a Wayne State study showed that pre-operative chemoradiation utilizing Fluorouracil (5-FU) and Mitomycin could induce complete pathologic responses in over 80% of patients, thus obviating the need for surgery (31). Numerous trials have established concurrent chemoradiation as superior to radiation alone (32,33). Surgical resection alone may still play a role in certain early-stage tumors with favorable characteristics. Surgery is sufficient with anal margin cancers in which the sphincter can be spared. Nonetheless, definitive chemoradiation is considered standard treatment by many institutions.
The advent of IMRT for anal cancer marked a considerable advance in treatment. Ongoing studies investigating the role of IMRT in anal cancer demonstrated promising clinical response rates with significantly better skin and normal organ toxicities as compared to conventional techniques (34). The pelvis is a tightly packed region of the body with numerous critical structures in intimate proximity to one another. Acute toxicities occur fairly frequently during treatment. Although some authorities suggest a dosimetric improvement of proton therapy over photon therapy in locally advanced anal cancer, very limited data are available for proton therapy in this disease. Traditional scanning beam techniques for proton therapy have field size limitations that make definitive proton treatment for anal cancer technically challenging. Intensity-modulated proton therapy (IMPT) techniques do not have the same field size limitations. IMPT may allow for treating anal cancer with protons with the potential of further decrease in adverse events, particularly late effects. Future studies should investigate ways to ensure adequate homogeneous coverage while sparing organs at risk.
Conclusions
Proton therapy shows great potential to increase therapeutic tolerance for patients with gastrointestinal malignancies. Numerous studies have demonstrated the capability to reliably reproduce the dosimetric quality of conventional conformal plans. Furthermore, improved beam conformality reduces the toxicity of surrounding organs at risk. This would lead to lower rates of late toxicity. Combined modality regimens have become the standard of treatment for a great majority of GI tract cancers. Reduction in radiation toxicity to organs at risk with proton therapy may allow the use of other systemic therapy or combination of therapies deemed too toxic when combined with conventional radiotherapy. Additionally, beam conformality with normal-tissue sparing becomes increasingly important in accordance with the general trend of finding ways to dose escalate. The possibility of decreasing radiation dose to organs at risk may also help facilitate chemotherapy dose escalation or allow for new chemotherapy combinations, which were previously deemed too toxic. The therapeutic ratio is the key parameter clinicians try to maintain in utilizing radiation therapy. Another major challenge for the future is proper identification of indications for proton therapy as a treatment modality. Proton centers are still relatively few in number; accordingly, outcomes are still fairly limited. Nonetheless, it is likely that the use of proton therapy will play a decisive role in the context of ongoing intensified combined modality treatments for GI cancers.
Acknowledgments
The author would like to thank William Preston, Ed.D for his assistance with manuscript preparation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Huan Giap and Eric Y Chuang) for the series “Particle Beam Therapy I” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.09.01). The series “Particle Beam Therapy I” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolff HA, Bosch J, Jung K, et al. High-grade acute organ toxicity as positive prognostic factor in primary radio(chemo)therapy for locally advanced, inoperable head and neck cancer. Strahlenther Onkol 2010;186:262-8. [PubMed]
- Wolff HA, Gaedcke J, Jung K, et al. High-grade acute organ toxicity during preoperative radiochemotherapy as positive predictor for complete histopathologic tumor regression in multimodal treatment of locally advanced rectal cancer. Strahlenther Onkol 2010;186:30-5. [PubMed]
- Wolff HA, Raus I, Jung K, et al. High-grade acute organ toxicity as a positive prognostic factor in primary radiochemotherapy for anal carcinoma. Int J Radiat Oncol Biol Phys 2011;79:1467-78. [PubMed]
- May KS, Yang GY, Khushalani NI, et al. Association of Technetium (99m) MAG-3 renal scintigraphy with change in creatinine clearance following chemoradiation to the abdomen in patients with gastrointestinal malignancies. J Gastrointest Oncol 2010;1:7-15. [PubMed]
- Cook MB, Wild CP, Everett SM, et al. Risk of mortality and cancer incidence in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 2007;16:2090-6. [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [PubMed]
- Jabbour SK, Thomas CR Jr. Radiation therapy in the postoperative management of esophageal cancer. J Gastrointest Oncol 2010;1:102-11. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:e345-51. [PubMed]
- Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2008;72:278-87. [PubMed]
- Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys 2011;81:1336-42. [PubMed]
- Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol 2011;2:39-44. [PubMed]
- Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1-11. [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [PubMed]
- Tian F, Appert HE, Myles J, et al. Prognostic value of serum CA 19-9 levels in pancreatic adenocarcinoma. Ann Surg 1992;215:350-5. [PubMed]
- Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48:1705-10. [PubMed]
- Kotowski A, Ma WW. Emerging therapies in pancreas cancer. J Gastrointest Oncol 2011;2:93-103. [PubMed]
- Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol 2011;2:157-67. [PubMed]
- Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol 1985;3:373-8. [PubMed]
- Shinchi H, Takao S, Noma H, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2002;53:146-50. [PubMed]
- Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg 2005;241:295-9. [PubMed]
- Brown MW, Ning H, Arora B, et al. A dosimetric analysis of dose escalation using two intensity-modulated radiation therapy techniques in locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys 2006;65:274-83. [PubMed]
- Kozak KR, Kachnic LA, Adams J, et al. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys 2007;68:1557-66. [PubMed]
- Bouchard M, Amos RA, Briere TM, et al. Dose escalation with proton or photon radiation treatment for pancreatic cancer. Radiother Oncol 2009;92:238-43. [PubMed]
- Terashima K, Demizu Y, Hashimoto N, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol 2012;103:25-31. [PubMed]
- Thomas PR, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol 1988;13:245-52. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Isacsson U, Montelius A, Jung B, et al. Comparative treatment planning between proton and X-ray therapy in locally advanced rectal cancer. Radiother Oncol 1996;41:263-72. [PubMed]
- Wolff HA, Wagner DM, Conradi LC, et al. Irradiation with protons for the individualized treatment of patients with locally advanced rectal cancer: a planning study with clinical implications. Radiother Oncol 2012;102:30-7. [PubMed]
- Nigro ND, Seydel HG, Considine B, et al. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983;51:1826-9. [PubMed]
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123-8. [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [PubMed]
- Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer 2007;6:348-56. [PubMed]


