
FOXC2 is overexpressed and participates in cell proliferation in ovarian cancer
Introduction
Ovarian cancer is one of the major gynecological malignancies with highest mortality (1). Due to the lack of specific symptoms and signs at early stage of the disease onset, most of the patients with ovarian cancer are diagnosed late, and about 75% of patients have extensive metastasis in the abdominal cavity when diagnosed (2). According to the statistical data, the 5-year survival rate of ovarian cancer could reach 90% if diagnosed early while it could be below 50% if cancer has migrated to the other tissues (3). Therefore, it is urgent to explore the molecular mechanism of ovarian cancer occurrence and development in order to search new targets for diagnosis and treatment of ovarian cancer.
Increasing evidences have shown that epithelial-to-mesenchymal transition (EMT) owns a close association with the acquisition of malignant characteristics in ovarian cancer cells, decreasing cell-to-cell contacts and enhancing invasive and migratory abilities (4-6). Forkhead box protein C2 (FOXC2) (also known as mesenchyme forkhead 1), is an EMT-inducing transcription factor and involved in cancer development (7). Overexpressed FOXC2 enhances the EMT and invasion progresses in ovarian cancer (8). Besides, FOXC2 also has many other effects on cancer progression. Sano et al. detected FOXC2 expression in vascular endothelial cells of melanoma, and angiogenesis as well as tumor growth were remarkably inhibited in the FOXC2+/− mutants, which suggests that FOXC2 plays an important role in tumor angiogenesis (9). Meanwhile, FOXC2 promoted colorectal cancer proliferation through inhibiting the activation of AKT and MAPK signaling pathways (10). Although recent studies have revealed several molecular mechanisms of FOXC2 in cancers, rare of them focus in ovarian cancer and the role of FOXC2 in ovarian cancer remains far from completely clear. Thus, more studies are needed to further explore the mechanisms of FOXC2 in ovarian cancer.
It was unknown how FOXC2 was expressed in ovarian cancer and whether FOXC2 could regulate ovarian cancer cell proliferation. In order to investigate these uncertainties, in this study, the expression and location of FOXC2 were detected and observed by immunohistochemistry, and the effect of FOXC2 on cell proliferation was also investigated by FOXC2-shRNA lentivirus interference in ovarian cancer.
Materials and methods
Samples
A total of 13 ovarian tissues (11 cases of epithelial ovarian cancer, one case of borderline serous cystadenoma and one case of normal ovarian tissue) were collected from November of 2013 to January of 2015 in First Hospital of Jilin University. All specimens were collected during surgery and washed with PBS for three times, followed by fixed in 10% neutral formalin more than 24 h. All the protocols were approved by the ethics committee of our hospital and informed consents were obtained from the participants.
Immunohistochemistry
The tissues were dehydrated with different concentrations of ethanol, transparented with xylene and subsequently embedded in paraffin blocks. Specimens were stored at 4 °C overnight, then were cut serially into slices of 3-5 µm thicknesses and stained by hematoxylin and eosin (H&E). Then the specimens were dried at 70 °C for 2 h in oven. After dewaxed and rehydrated, the tissue sections were blocked with horse serum, followed by incubated with FOXC2 active antibody (1:100, Abcam) at 4 °C overnight. After extensive washed with PBS for three times, sections were incubated with biotinylated secondary antibody (1:100, Abcam) at room temperature for 30 min. The 3,3’-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA) was used as the final chromogen, and hematoxylin was utilized as the nuclear counterstain. Ten visual fields were randomly selected under light microscope and all experiments were performed three times.
Construction of FOXC2-shRNA lentiviral vector (LV)
The shRNAs targeting FOXC2 mRNA (NCBI Reference Sequence: NM_013519.2) were designed online:
- shRNA-FOXC2-1, 5'-CCACACGTTTGCAACCCAA-3';
- shRNA-FOXC2-2, 5'-CAAGAGGTCTCTCCGGATAAG-3';
- shRNA-NC: 5'-TTCTCCGAACGTGTCACGT-3'
The lentivirus expression plasmid (pLenR-GPH or pGC-LV-control vector), together with pRsv-REV, pMDlg-pRRE and pMD2G virus packaging plasmids, were co-infected into 293T cells (Chinese Academy of Sciences Shanghai cell institute) with lipofectamine 2000 (Invitrogen) as the manufacturer’s instructions for the preparation of FOXC2-shRNA lentivirus. After 48 h of infection, lentivirus was harvested and centrifuged to remove cell debris followed by filtering using 0.45 µm cellulose acetate filters. Then the lentivirus was performed ultracentrifugation at speed of 25,000 rpm/min at 4 °C for 2 h. Finally, the virus was resuspended in 500 µL DMEM and virus titer was detected by interfered 293T cells.
Cell culture and viral infection
Mouse ovarian cancer cell, ID8, was provided by Cancer Institute of Second Military Medical University. ID8 was cultured in H-DMEM supplemented with 5% FBS (Invitrogen) and maintained at 37 °C humidified chambers containing 5% CO2. The cells were divided into four groups, control group, control lentivirus (LV-NC) group, LV-FOXC2-1 group(ID8 cells infected with FOXC2-shRNA1 LV) and LV-FOXC2-2 group (ID8 cells infected with FOXC2-shRNA2 LV). Cells in LV-FOXC2-1 and LV-FOXC2-2 groups were infected with 1 µL of shRNA-viruses (1.2×108~1.3×108 Tu/mL of each virus) for 72 h.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using Trizol reagent (BD Biosciences, Franklin Lakes, NJ, USA). Reverse transcriptase and oligo (dT) were used to preform reverse transcription (PrimeScript RT reagent Kit). The real-time RT-PCR procedure was conducted as following: 95 °C for 10 min; 40 cycles of 95 °C for 15 sec, 60 °C for 20 sec and 72 °C for 20 sec. Levels of mRNA were conducted by using a SYBR Green Real-time PCR Master Mix (TaKaRa, Shiga, Japan) on iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Relative mRNA expression level was calculated using the 2-ΔΔCt method and normalized to GAPDH. The primers were as follows: FOXC2 Forward: 5'-GCCACCTCCTGGTATCT; FOXC2 Reverse: 5'-GGACAGCCTCAGTATTTGGTG-3'; GAPDH Forward: 5'-AAATCCCATCA CCATCTTCC-3'; GAPDH Reverse: 5'-TCACACCCATGACGAACA-3'.
Western-blot analysis
Total proteins were extracted by using cold lysis buffer containing 50 µL 10 mg/mL phenylmethanesulfonyl fluoride (PMSF) and the concentrations of protein samples were determined according to BCA protein assay kit (Sigma, St. Louis, MO, USA). Then the protein samples (100 µL) were mixed with 20 µL × 5 loading buffer and denatured at 100 °C for 5 min. The 10% SDS-PAGE was performed to separate protein samples and polyvinylidene fluoride (PVDF) membrane was chosen for transferring. The membranes were blocked with 3% BSA for 1 h at room temperature, followed by incubated with anti-FOXC2 or anti-GAPDH (1:1,000; Sigma) overnight at 4 °C. After washed with PBS for three times, the membranes were then incubated with the anti-rabbit IgG (1:1,000; Sigma) at room temperature for 1 h. Finally, the immunoreactive bands were visualized by chemiluminescence and detected through an enhanced chemiluminescence (ECL) detection system (Amersham, Buckinghamshire, UK). The images were captured on X-ray films, and scanned for quantification using Image software.
Cell proliferation assay [CCK8Cell counting kit-8 (CCK8) assay]
CCK8 (Beyotime Biotechnology) assay was conducted to examine cell proliferation as the manufacturer's instructions. Briefly, ID8 cells were seeded with a density of 5×104 cells in a 96 well cell culture plate with 100 µL medium and grew to receive a density of 70%. Then the cells were washed with PBS and mixed with fresh medium containing 10 µL CCK8. The cells were reacted at dark cell lined with foil for 3 h. Then the supernatant of each well were transferred into a new 96 well plate and the absorbance was measured at 450 nm using spectrophotometer at 24, 48, 72 and 96 h. The CCK8 reagent contains WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt], which can be reduced by dehydrogenases after entering cells and then produce orange colored formazan products. There is directly proportional relationship between the amount of formed formazan and the number of living cells.
Statistical analysis
All data was analyzed using SPSS version 19.0 (SPSS Inc, Chicago, IL, USA). Chi-square test was performed for the comparisons of categorical variables, student’s t-test and Analysis of variance were performed for normally distributed continuous variables, respectively. P<0.05 was considered to be statistically significant.
Results
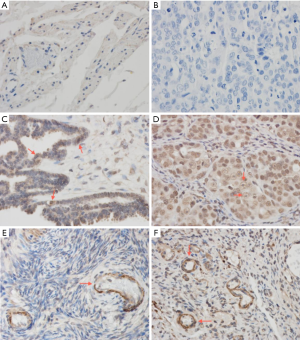
FOXC2 over-expressed in ovarian cancer tissues
FOXC2 expression in normal, borderline serous cystadenoma and ovarian cancer cells are shown in Figure 1 by immunohistochemistry assay. No expression of FOXC2 was found in normal ovarian tissue (Figure 1A), which was similar with the tissue without stained with anti-FOXC2 (Figure 1B). In borderline serous cystadenoma, FOXC2 was expressed on vascular wall including vascular wall of endocrine glands, as red arrow indicated in Figure 1C. In epithelial ovarian cancer tissue, FOXC2 was strongly expressed and mainly expressed in the nucleus of tumor cells, with nuclear volume increasing, irregular in shape and some visible mitotic (Figure 1D). As shown in Figure 1E,F, FOXC2 was mainly expressed in cytoplasm of vascular endothelial cells of ovarian cancer and borderline ovarian tumor stromal.
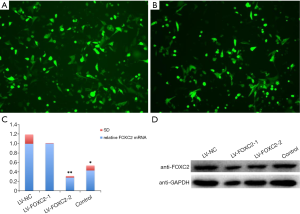
Successful construction of interference lentivirus
The infection efficiency reached to 70% when ID8 cells were infected with 1.0 µL LV-FOXC2 lentivirus particles for 72 h (Figure 2A,B). The mRNA and protein expression levels of FOXC2 were significantly decreased in ID8 cells infected with LV-FOXC2, especially in LV-FOXC2-1 group (Figure 2C,D). According to the results, LV-FOXC2-1 with the better interference effect than LV-FOXC2-2 was chosen for the following experiment.
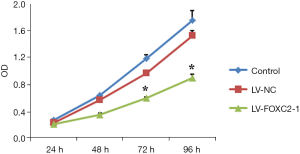
FOXC2-shRNA inhibited cell proliferation
ID8 cells were infected with LV-FOXC2-1 to examine the cell proliferation (Figure 3). At 24 h after adding CCK8, no significant difference of cell proliferation was found among control, LV-NC and LV-FOXC2-1 groups (P>0.05). However, after 48h adding CCK8, cell proliferation was significantly suppressed in cells of LV-FOXC2-1 group than that in control and LV-NC groups in a time-dependent manner (P<0.05).
Discussion
FOXC2 is known to be involved in mesenchymal cell development during embryogenesis and closely associated cancer metastasis as an EMT-inducing TF (7,11). Increasing evidences have confirmed that FOXC2 was upregulated in some invasive and metastatic cancers, such as breast cancer, esophageal squamous cell carcinoma and lung cancer (12-14). In this study, overexpression of FOXC2 was also certified by immunohistochemistry in borderline serous cystadenoma and ovarian cancer.
Angiogenesis plays a pivotal role in the occurrence and development of tumor and nodal metastasis (15,16). FOXC2 has been implicated in vascular development (17) and FOXC2 could indirectly modulate angiogenesis, remodeling, vascular patterning by regulating Ang-2 expression (18). It has been reported that the expression of FOXC2 gene was increased with malignancy, especially with invasion degree and blood vessel hyperplasia. The role of FOXC2 in angiogenesis has been reported in several cancers, including melanoma cells, oral squamous cell carcinoma and breast cancer (9,19). FOXC2 has been demonstrated to induce the transcription of integrin β3 and chemokine receptor CXCR4, two cell surface proteins of vascular endothelial cells, by activating their promoters via FOXC-binding elements in nuclear (20,21). Furthermore, integrin β3 and CXCR4 are essential for the migration of endothelial cell, a critical process for angiogenesis (22,23). Our immunohistochemistry results showed that FOXC2 was detectable in the nuclear of epithelial ovarian cancer tissue, and FOXC2 expression was also found in interstitial vessels of ovarian cancer. Positive expression of FOXC2 was found in borderline tumor vascular endothelial cells and stromal cells, which might indicate borderline tumor cells differentiate toward canceration, invasion and metastasis by interstitial vascular invasion. Therefore, overexpression of FOXC2 might have effects on EMT process and it might be used as a biomarker for the malignant transformation of borderline ovarian tumors, which was still needed to be certified in further studies.
Increasing researches revealed that FOXC2 owns influences on cell proliferation in several cancers. Cui et al. reported that FOXC2 promoted cell proliferation through up-regulating cyclin D1 and p-FOXO3a as well as down-regulating p27 via activating MAPK and AKT pathways. Furthermore, nuclear translocation of FOXC2 is necessary for its upregulation of cell proliferation (10). Additionally, targeted silencing of FOXC2 is effective to reduce invasion potentiality as well as cell proliferation (24). Li and his colleagues have demonstrated that glioblastoma cells proliferation was significantly increased when FOXC2 was ectopic expression, while it was significantly decreased after interfered with siRNA targeting FOXC2 (25). In our study, cell proliferation ability of ID8 cells was significantly decreased after FOXC2 silence by shRNA lentivirus, which suggested that FOXC2 expression was closely associated with the ID8 cells proliferation. Specific molecular mechanisms of FOXC2 on ovarian cancer cell proliferation were still unclear. Another member of FOX family, FOXM1 expression is elevated in multiple human cancers including ovarian cancer, and previous studies have suggested that FOXM1 plays essential roles in cell cycle progression and cancer cell growth via phosphorylated by MELK (26). Considering FOXC2 and FOXM1 were all belong to FOX family and overexpressed in ovarian cancer, they might have the similar mechanism in cell proliferation, which still need further clarificaton.
Conclusions
In this study, we demonstrated that FOXC2 was overexpressed and mainly expressed in the cytoplasm of vascular endothelial cells in vascular wall of ovarian cancer and borderline ovarian tumor stroma while FOXC2 was also strongly expressed in nucleus of ovarian cancer. Cell proliferation was significantly suppressed in cells with FOXC2 silencing. Thus, targeting FOXC2 may represent a potential therapeutic benefit for ovarian cancer. The results obtained in our study might help to gain further knowledge and insights into molecular mechanism of tumorigenesis as well as therapy in ovarian cancer.
Acknowledgments
Funding: This work was supported by Jilin province science and technology development project (grant number 20110477)and Jilin province industrial technology research and development project (grant number 2011007-10).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hsinyu Lee and Markus H. Gräler) for the series “Lysophospholipids on Immunity and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.10.04). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Su D, Katsaros D, Xu S, et al. ADP-ribosylation factor-like 4C (ARL4C), a novel ovarian cancer metastasis suppressor, identified by integrated genomics. Am J Transl Res 2015;7:242-56. [PubMed]
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol 2009;112:265-74. [PubMed]
- Koutsaki M, Spandidos DA, Zaravinos A. Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: prognostic value and prospective role in ovarian cancer therapeutics. Cancer Lett 2014;351:173-81. [PubMed]
- Comamala M, Pinard M, Thériault C, et al. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer 2011;104:989-99. [PubMed]
- Cheng JC, Auersperg N, Leung PC. TGF-beta induces serous borderline ovarian tumor cell invasion by activating EMT but triggers apoptosis in low-grade serous ovarian carcinoma cells. PLoS One 2012;7:e42436 [PubMed]
- Ren YH, Liu KJ, Wang M, et al. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget 2014;5:7093-104. [PubMed]
- Liu B, Han SM, Tang XY, et al. Overexpressed FOXC2 in ovarian cancer enhances the epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Oncol Rep 2014;31:2545-54. [PubMed]
- Sano H, Leboeuf JP, Novitskiy SV, et al. The Foxc2 transcription factor regulates tumor angiogenesis. Biochem Biophys Res Commun 2010;392:201-6. [PubMed]
- Cui YM, Jiang D, Zhang SH, et al. FOXC2 promotes colorectal cancer proliferation through inhibition of FOXO3a and activation of MAPK and AKT signaling pathways. Cancer Lett 2014;353:87-94. [PubMed]
- Mani SA, Yang J, Brooks M, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A 2007;104:10069-74. [PubMed]
- Hollier BG, Tinnirello AA, Werden SJ, et al. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res 2013;73:1981-92. [PubMed]
- Nishida N, Mimori K, Yokobori T, et al. FOXC2 is a novel prognostic factor in human esophageal squamous cell carcinoma. Ann Surg Oncol 2011;18:535-42. [PubMed]
- Yu YH, Chen HA, Chen PS, et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 2013;32:431-43. [PubMed]
- Sasahira T, Kirita T, Kurihara M, et al. MIA-dependent angiogenesis and lymphangiogenesis are closely associated with progression, nodal metastasis and poor prognosis in tongue squamous cell carcinoma. Eur J Cancer 2010;46:2285-94. [PubMed]
- Wang J, Du Y, Liu X, et al. MicroRNAs as Regulator of Signaling Networks in Metastatic Colon Cancer. Biomed Res Int 2015;2015:823620.
- Papanicolaou KN, Izumiya Y, Walsh K. Forkhead transcription factors and cardiovascular biology. Circ Res 2008;102:16-31. [PubMed]
- Xue Y, Cao R, Nilsson D, et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci U S A 2008;105:10167-72. [PubMed]
- Sasahira T, Ueda N, Yamamoto K, et al. Prox1 and FOXC2 act as regulators of lymphangiogenesis and angiogenesis in oral squamous cell carcinoma. PLoS One 2014;9:e92534 [PubMed]
- Hayashi H, Kume T. Forkhead transcription factors regulate expression of the chemokine receptor CXCR4 in endothelial cells and CXCL12-induced cell migration. Biochem Biophys Res Commun 2008;367:584-9. [PubMed]
- Hayashi H, Sano H, Seo S, et al. The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression. J Biol Chem 2008;283:23791-800. [PubMed]
- Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 2007;28:299-307. [PubMed]
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 2008;8:604-17. [PubMed]
- Zheng CH, Quan Y, Li YY, et al. Expression of transcription factor FOXC2 in cervical cancer and effects of silencing on cervical cancer cell proliferation. Asian Pac J Cancer Prev 2014;15:1589-95. [PubMed]
- Li W, Fu X, Liu R, et al. FOXC2 often overexpressed in glioblastoma enhances proliferation and invasion in glioblastoma cells. Oncol Res 2013;21:111-20. [PubMed]
- Joshi K, Banasavadi-Siddegowda Y, Mo X, et al. MELK-dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells. Stem Cells 2013;31:1051-63. [PubMed]


