
Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: the fat’s in the fire
Introduction
A link between inflammation and carcinogenesis has been appreciated for over a century, beginning with the recognition by German physician and scientist Rudolf Virchow that cancer behaves like ‘a wound that does not heal’ (1,2). Cancers sometimes arise at sites of chronic inflammation, and tumors often contain inflammatory infiltrates. In addition, elevated inflammatory markers and cytokines accompany many malignant conditions (3-5).
Despite recognition that inflammation and carcinogenesis are somehow connected, the mechanistic underpinnings of this important relationship remain elusive. In particular, our knowledge regarding which inflammatory signals contribute to cancer and how inflammatory signals influence the evolution of cancer from normal tissue remains incomplete. There is abundant evidence confirming that inflammation can promote the progression of many forms of cancer by enhancing angiogenesis, metastasis, metabolic advantages and chemotherapy resistance (6-8). In addition, an emerging body of data has begun to implicate inflammation in the earliest stages of carcinogenesis, including the seminal process of cell transformation, during which a cell acquires the fundamental characteristics of a cancer cell, namely the ability to proliferate in the absence of growth signals, exhibit anchorage-independent growth and form tumors in immunocompromised mice (9). Additional studies have shown that under certain circumstances inflammation may alter the epigenetic landscape, influence microRNA expression and even promote mutagenesis (10). Thus, recent findings are beginning to dispel the long-held notion that mutations are the ‘match that lights the fire’ of cancer, whereas inflammation is merely the ‘fuel that feeds the flame’ (11). Instead, inflammation is increasingly being viewed as a potentially seminal event in some types of carcinogenesis.
Nowhere is the link between inflammation and carcinogenesis more firmly substantiated than in colitis-associated colon cancer (CAC). CAC is a pathological condition defined by the development of colon cancer in patients afflicted by Crohn’s disease (CD) or ulcerative colitis (UC), two inflammatory diseases of the gut which together comprise the disease category called inflammatory bowel disease (IBD). Patients with IBD involving the colon exhibit an increased risk of developing colon cancer compared to the general population (12). In patients with CD, the risk of small-bowel adenocarcinoma is 20 to 30 times that in patients without CD (13). The risk of CAC correlates with the intensity, extent and duration of inflammation, with pediatric IBD patients being at particular risk due to the long duration of their inflammatory illness (12). For example, teenagers suffering with pancolitis have a lifetime risk of developing colorectal cancer that is greater than 15% (12). On the other hand, the latency between IBD onset and CAC development provides a unique opportunity for therapeutic and chemopreventive interventions that could halt or reverse the inflammatory processes that contribute to carcinogenesis.
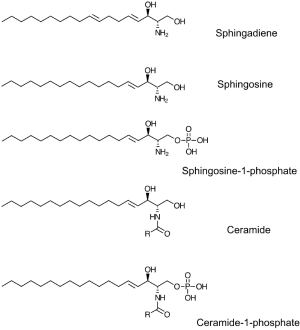
Sphingolipids represent a ubiquitous class of lipids found in plants, animals, fungi and some bacteria. As such, they are present in human cells, tissues, and extracellular fluids. In addition, sphingolipids derived from various food products are normal constituents of the human diet (14). The phospholipid metabolite sphingosine-1-phosphate (S1P) is the final common product generated by the breakdown of mammalian sphingolipids (Figure 1). S1P is found at high levels in the lymph and the bloodstream, where it circulates bound to high-density lipoprotein and albumin (15). S1P serves as a ligand for a family of five G protein-coupled receptors (S1P1-5) implicated in many physiological functions, including brain and vascular development, blood vessel permeability, the trafficking of lymphocytes and other hematopoietic cells, and innate immunity (16). S1P signaling through the S1P receptor (S1PR) S1P1 is essential for lymphocyte egress from the thymus and peripheral lymphoid organs (17). Pharmacological agents that either target S1P1 or disrupt S1P chemical gradients induce lymphopenia without blocking other lymphocyte function (18). These agents have shown efficacy in the treatment of autoimmune diseases including multiple sclerosis, rheumatoid arthritis and IBD (see below) (19).
S1P signaling has also been implicated in the activation of two critical transcription factors, nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3), which independently regulate the transcription of large sets of gene targets involved in inflammatory signaling, cell proliferation and cell death (20,21). NF-κB and STAT3 operate as central signaling nodes linking inflammation and carcinogenesis. By modulating the activities of these critical molecular targets, S1P sits at a hub of intracellular activities, poised to influence inflammation and carcinogenesis. Not surprisingly, recent studies have implicated S1P signaling and metabolism in the pathogenesis of IBD and CAC.
In this review, we will discuss the evidence implicating S1P signaling and metabolism in IBD and CAC. We will summarize current knowledge linking these processes and describe in greater detail our own recent study demonstrating enhanced IBD and CAC in genetically engineered mice that accumulate S1P in gut tissues due to disruption of the main enzyme responsible for S1P degradation (22). The results of our study show that S1P promotes inflammatory responses, cell transformation, IBD development, and the progression of IBD to CAC. Our findings demonstrate that these effects are mediated through the impact of S1P on STAT3 signaling. Further, our results suggest that dietary sphingolipids may exacerbate or prevent IBD and CAC depending on their ability to be converted to the pro-inflammatory lipid, S1P. Unanswered questions and potential new areas of study related to S1P metabolism and signaling will also be discussed.
IBD and colitis-associated colon cancer
CD and UC are two conditions that involve unpredictable and destructive chronic inflammatory processes that make up the disease group called IBD (23). It is estimated that IBD currently affects over one million persons in the US, including 1 in 10 persons under the age of 18 (24). UC is associated with inflammation of the mucosa of the colon and rectum. In CD, the full thickness of the bowel wall is inflamed, and any part of the digestive tract from the mouth to the anus may be involved. IBD is associated with weight loss, diarrhea, abdominal pain and fever (25-27). It can lead to fistulas, perforations, infections and can stunt the growth and pubertal development of affected children. Microscopic features of IBD include ulceration, abscesses, inflammatory infiltrates, edema, mucin depletion and loss of the normal crypt architecture. In addition to these complications, when IBD involves the colon, patients are faced with an increased risk of developing intestinal dysplasia and CAC (10).
Standard IBD treatments include steroids, other immunosuppressants, aminosalicylates and antibiotics. Other medications are aimed at reducing symptoms and maintaining nutrition. For refractory cases and/or complications, surgery may be required. Critical obstacles in treating IBD and preventing CAC include the need for ideal surrogate markers of tissue inflammation, safe agents for preventing IBD in the public, the elderly and the young, and safe, effective alternatives to highly toxic or unpleasant treatments including steroids, other immunomodulatory agents, and elemental diets. Targeted therapies and dietary chemoprevention strategies to reduce the need for global immunosuppressive regimens in IBD patients are urgently needed.
IBD is caused by dysregulation of homeostatic mechanisms that normally maintain the optimal gut microbiome, intestinal mucosal integrity and function (23,27-44). Genome-wide association studies have implicated a number of human genes as risk factors for IBD, including ATG16L, NOD2/CARD15, IBD5, CTLA4, TNFSF15, JAK2, STAT3, IL23R, and ORMDL3 (45). The function of these genes has provided some clues regarding the etiology of IBD, pointing to the role of antimicrobial peptides, innate and adaptive immune cell function, Th-17 cells, regulatory T cells (Tregs), and cytokines (tumor necrosis factor, interleukins 17, 23, 12, 22 and IL-6) as contributing factors in IBD. Many of these cytokines serve as ligands for cell surface receptors that activate NF-κB and STAT3, two key transcriptional regulators that control cell growth, programmed cell death pathways and inflammation in response to intrinsic and environmental stimuli. In addition to genes directly involved in inflammation and the innate immune response, genome-wide association studies have identified IBD risk genes implicating autophagy and endoplasmic reticulum (ER) stress in the development of IBD. One ER-stress related gene implicated in IBD risk, ORMDL3, is a member of the ORM class of proteins, which have been shown to act as negative regulators of sphingolipid biosynthesis (46,47). This finding, along with other studies in mouse models of IBD and CAC, point toward a role for sphingolipids—and specifically for S1P signaling —in these diseases.
Sphingolipids in immune functions, inflammation and cancer
Sphingolipids are ubiquitous membrane lipids found in our bodies as well as in our diet (48,49). In addition to serving structural roles in cell membranes such as the formation of lipid rafts, sphingolipid turnover yields metabolites that regulate cell proliferation, migration and programmed cell death. In so doing, sphingolipids influence processes that are critical to the initiation and progression of cancer, and they have been implicated in both early and late stages of carcinogenesis (50).
All sphingolipids are built upon a long chain amino base backbone, which in mammals is sphingosine (Figure 1). Gut enterocytes synthesize sphingolipids de novo and also import and metabolize dietary sphingolipids. Degradation of mammalian sphingolipids by enzymes located in the brush border of the lower gastrointestinal (GI) tract results in release of sphingosine, which enters enterocytes and can be incorporated into complex sphingolipids (48). Alternatively, sphingosine can be converted to S1P by phosphorylation at its C1 carbon, a biochemical step catalyzed by either of two sphingosine kinases (SKs), SphK1 and SphK2 (51). S1P can be dephosphorylated to regenerate sphingosine, a process catalyzed by either of two specific S1P phosphatases, Sgpp1 and Sgpp2, as well as by members of the LPP class of nonspecific lipid phosphatases (51). S1P is irreversibly degraded to hexadecenal and ethanolamine phosphate by the intracellular, pyridoxal 5’-phosphate (PLP)-dependent enzyme sphingosine phosphate lyase (SPL) (52). Despite the fact that S1P is a substrate for many lipid phosphatases, SPL alone is responsible for regulating steady state cellular, tissue and extracellular S1P levels, as shown by the profound elevation of S1P observed in SPL-deficient cell and animal model systems (22,53,54).
Upregulation of SphK1 has been shown to occur in mouse models of colon cancer and in human colon adenomas and colorectal cancer specimens (55,56). Evidence derived from SphK1 knockout (KO) mice and cellular experiments with SphK1 silencing demonstrate that SphK1 and production of S1P promote colon cancer progression (55,56). Conversely, downregulation of SPL in adenomas of ApcMin/+ mice (which exhibit activated Wnt signaling and develop florid intestinal polyposis) correlates with accumulation of S1P in intestinal adenomas, compared to the low levels found in surrounding non-tumor intestinal tissue (57). SPL downregulation has also been observed in human colorectal and prostate cancers (57,58). In the case of prostate cancers, SPL expression was inversely correlated with SphK1 expression, and both SPL downregulation and SphK1 upregulation correlated with higher clinical stage and poor prognosis (58). Further, SPL overexpression sensitized both colon and prostate cancer cells to DNA damaging chemotherapy (57,58). These cumulative findings suggest that changes in S1P metabolism contribute to cancer development and can influence the response to treatment. Further, these studies suggested that proteins involved in S1P metabolism, transport and signaling may be particularly useful therapeutic targets in cancers exhibiting long latency periods that are amenable to chemopreventive strategies.
In 2004, Jason Cyster’s research group showed that T lymphocytes require S1P1 expression and signaling through S1P1 at the last stage of their maturation in order to egress from the thymus and enter the circulation (17). The role of S1P1 in lymphocyte egress explains the lymphopenia that occurs with administration of FTY720 (fingolimod), a receptor agonist that induces receptor desensitization, accounting for its S1P1 functional antagonistic property (59-62). Additional studies have shown that administration of pure S1P1 antagonists also results in lymphopenia (63). Subsequently, Cyster’s group showed that inhibiting SPL also causes a block in lymphocyte egress, in this case by preventing formation of an S1P chemical gradient that stimulates chemotaxis of mature thymocytes into the bloodstream (64). Similar events were shown to orchestrate the exit of T lymphocytes from peripheral lymphoid organs. SKs located in perivascular cells at the corticomedullary junction region of the thymus are the source of the S1P that stimulates lymphocyte egress (65). Graler and colleagues showed that SphK2 specifically contributes to the redistribution of S1P in lymphoid tissues and facilitates lymphocyte egress (66). Importantly, administration of S1P1 antagonists has been shown to reduce progression of autoimmune diseases, with the most well established effects being in chronic relapsing multiple sclerosis (67). These profoundly important studies have provided the first clinical context in which targeting S1P signaling and metabolism has improved patient outcomes and has been adopted into medical treatment for a human disease. These studies raise the possibility that similar S1P-targeted strategies could be of potential benefit in other inflammatory diseases including rheumatoid arthritis, muscular dystrophy, IBD, and to prevent transplant-related complications such as graft vs. host disease.
In addition to its role in the adaptive immune response, S1P signaling has been shown to contribute to innate immune functions including mast cell functions, monocyte and macrophage activity, T-reg functions, and cytokine signaling (68). Recent studies have also provided evidence linking S1P signaling to two key inflammatory signaling pathways regulated by the transcription factors NF-κB and STAT3. In 2010, the research group of Hua Yu demonstrated that the S1P and STAT3 signaling pathways co-stimulate one another in a regulatory loop that promotes carcinogenesis (21). In this system, STAT3 upregulates one of its target genes, S1P1, thereby allowing S1P to induce a signal that results in STAT3 activation. The amplification of these pathways by mutual co-stimulation was shown to produce a constitutively active signal that contributes to cancer progression and metastasis. Also in 2010, Spiegel and colleagues demonstrated that S1P is a cofactor required by TRAF2 to activate NF-κB in response to TNFα stimulation (20). The elucidation of these critical connections, combined with the revelations regarding S1P’s role in hematopoietic cell trafficking, further established that S1P signaling is an important inflammatory pathway that contributes to immune function and has a potential role in the pathogenesis of inflammation-associated diseases.
S1P signaling and metabolism in IBD and CAC
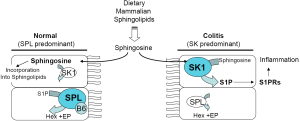
Sphingolipids are found in many dietary food sources, and the enzymes required for sphingolipid degradation and reutilization are found in the brush border of the intestinal mucosa as well as in the epithelial cells of the gut (14,48). However, evidence from a variety of sources suggests that the enzymes of S1P metabolism may not be static, and in fact may be significantly responsive to inflammatory conditions in the gut. Under homeostatic conditions, S1P is detected only at low levels in gut epithelial tissues (Figure 2). This can be explained in part by the low expression of SphK1 and SphK2 in healthy colon tissues (56,69,70). Under normal circumstances any S1P generated from endogenous or dietary sphingolipids is likely to be immediately degraded by SPL, which is highly expressed and active in the differentiated enterocytes of healthy small and large intestinal mucosa (71). However, the situation is quite different when inflammation is present. SphK1 is upregulated and activated in colonic tissues in mouse models of both IBD and CAC (72-75). Consistent with this finding, we have observed high SphK1 and S1PR expression levels in the colon tissues of pediatric IBD patients compared to controls (Degagne, unpublished observations). The upregulation of SphK1 enhances S1P production and accumulation. Although changes in SPL activity have not been reported in IBD or CAC, the enzyme’s cofactor, PLP, also known as vitamin B6, has been found at reduced levels in inflamed murine colon tissues, in inflammatory conditions in humans, and in IBD patients during flare compared to in remission (76,77). This raises the possibility that SPL activity may be compromised under inflammatory conditions, which would further enhance S1P accumulation.
Based on the positive effects of targeting S1PRs in other autoimmune diseases, a number of research groups have investigated the potential utility of applying this strategy in the treatment of IBD (78). Studies employing S1PR antagonists including FTY720 (which targets S1P1, 3, 4 and 5), and W-061 and KRP-203 (which both target S1P1) have been undertaken in a variety of different mouse models of IBD (79-84). These models include the IL-10 KO mouse, chemical induction of IBD with dextran sodium sulfate (DSS), 2,4,6-trinitrobenzenesulfonic acid or oxazolone, and the adoptive transfer model, all of which have yielded promising results. These represent a heterogeneous set of IBD model systems including CD vs. UC, T cell-dependent and -independent conditions, and models involving predominantly Th1 or Th2 immune responses. In addition to targeting S1PRs, genetic and pharmacological approaches to reduce S1P biosynthesis have also been shown to attenuate colitis in murine models of IBD. These include genetic disruption of SphK1 and treatment of mice with pharmacological inhibitors of SK activity with ABC747080 and ABC294640 (72). The positive results of targeting S1P using these diverse models strongly support the notion that targeting S1P signaling and metabolism may be of benefit in IBD.
S1P signaling may also be important in driving the development of CAC as a consequence of IBD. Spiegel’s group showed that when mice lacking the secondary sphingosine kinase (SphK2) and control mice were treated with a chemical regimen that induces CAC, the KO mice exhibited increased IBD symptoms, as well as an increase in CAC, as shown by higher tumor incidence, tumor size and tumor burden than controls (70). They found that SphK2 KO mice exhibited elevated expression of SphK1. The production of S1P by SphK1 in these mice leads to a persistent amplification of a signaling pathway involving NF-κB, IL-6, STAT3 and S1P1 in chronic colitis and in CAC. CAC development was blocked by treatment with FTY720, which functionally antagonizes S1PRs and also exhibits inhibitory activity against SphK1.
Generation of a gut-specific conditional SPL KO mouse (SPLGutKO)
SPL is highly expressed in differentiated enterocytes and is downregulated in murine adenomas and spontaneously arising colorectal cancers in humans (57,71). SPL provides a critical function in regulating S1P levels and actions in the lower GI tract. By preventing S1P accumulation in enterocytes, which are constantly exposed to DNA-damaging oxidants in the gut lumen, SPL likely helps to promote apoptosis and maintain their high turnover rate. We considered that SPL KO mice might afford an opportunity to examine whether S1P metabolism plays a role in IBD and CAC. However, global SPL deletion in mice results in cellular and tissue S1P levels hundreds of times higher than normal, a state that is not compatible with life (our unpublished findings) (85). Therefore, to study the role of SPL and S1P signaling in IBD and CAC, we generated an inducible intestinal-specific KO line (22). This was accomplished by generating mice with “floxed” alleles of Sgpl1 which encodes murine SPL, and then breeding these mice to mice harboring a Cre transgene (Tg) under control of an intestinal epithelium-specific promoter. In the resulting mouse line, which we have called the SPLGutKO, recombination and inactivation of Sgpl1 is induced specifically in tissues of the intestinal epithelium upon treatment with the antibiotic β-naphthoflavone (NF) (86). This results in virtual absence of SPL activity in gut tissues and produces an 8-fold increase in S1P levels in tissues of the lower GI tract including the colon. In contrast, no significant changes in the tissue levels of sphingosine, ceramide, dihydrosphingosine or sphingomyelin were observed in the SPLGutKO intestinal tissues. To avoid differences in the microbiome of SPLGutKO and control mice caused by antibiotic treatment, “floxed” littermates lacking the Cre Tg were treated with NF and used as controls.
IBD and CAC are enhanced in the SPLGutKO mouse
We found that SPLGutKO and control mice were similar with regard to body weight, litter sizes, and life spans. Further, colitis and CAC did not develop spontaneously in SPLGutKO (or control) mice. Therefore, to determine the impact of SPL downregulation on IBD and CAC, we compared the sensitivity of SPLGutKO and control mice to a well-established regimen for inducing CAC using the gut irritant DSS combined with the mutagen azoxymethane (AOM). Disease activity was monitored by following IBD symptoms of diarrhea, blood in stools and weight loss. Under these conditions, we observed more profound symptoms of IBD in SPLGutKO mice compared to controls. Consistent with these findings, plasma and colon cytokines associated with IBD in humans, including TNF-α, IL-1β, IL-6, IL-17, IL-21 and IL-23, were elevated to a greater degree in SPLGutKO mice than controls. Gross and microscopic pathology revealed more profound IBD pathology in SPLGutKO mice, including increased recruitment of macrophages to colon tissues, shorter colon lengths due to fibrosis and tissue constriction, and lower hematocrits and larger spleen sizes, indicating blood loss in stool and compensatory secondary hematopoiesis in the spleen. In addition to suffering increased IBD symptoms and pathology, SPLGutKO mice developed more colitis-associated tumors than control mice. Further, the uninvolved tissues of their colons exhibited a higher rate of proliferating cells per crypt than controls. In contrast, we observed no difference in the size of tumors in both animal groups, indicating that in this model system tumor progression was not significantly affected by S1P signaling. These cumulative findings suggested that SPL disruption in the lower GI tract worsens colitis and promotes the development of CAC by influencing an early stage in the carcinogenic process.
Enhanced IBD and CAC in SPLGutKO mice is mediated by STAT3 signaling
STAT3 signaling promotes colon cancer cell proliferation, is constitutively activated in most colon cancers, is implicated in IBD pathogenesis, and participates in a positive feedback loop with S1P signaling (21,45,87-92). Therefore, we suspected that the impact of SPL downregulation on IBD and CAC might be mediated through the IL-17/STAT3 signaling pathway. To probe for differences in the milieu from which CAC tumors arise in SPLGutKO and control mice after AOM/DSS, we performed immunohistochemistry, immunofluorescence and western blotting on the non-tumor tissues from these mice. We demonstrated a significantly higher level of STAT3 activation in the non-tumor colon tissues of SPLGutKO mice compared to control mice. STAT3 activation was higher in the differentiated colonic enterocytes of SPLGutKO mice, which showed much higher staining than controls, whereas STAT3 activation levels in tumor tissues and in interstitial tissues were comparable in both groups. We also observed upregulation of STAT3 target genes including S1P1 in murine tissues and in cells deficient in SPL. In addition to regulating conventional mRNA target expression, STAT3 has been shown to induce a number of microRNAs, including miR-21 and miR-181b1, which serve a crucial role in mediating cell transformation downstream of STAT3 via their ability to silence the two anti-oncogenes PTEN and CYLD, respectively (93). Interestingly, the non-tumor tissues of SPLGutKO mice exhibited upregulation of miR-21 and miR-181b1 and downregulation of their own target proteins PTEN and CYLD compared to control mice. Our findings suggested that SPL disruption enhances STAT3 signaling and promotes induction of STAT3-activated microRNAs important in cell transformation. When a STAT3 inhibitor was administered to SPLGutKO mice concomitant with AOM/DSS treatment. The features of IBD and CAC were attenuated, including elevated inflammatory cytokines, IBD pathology and tumor incidence. In addition, miR-21 and miR-181b1 levels were reduced, whereas PTEN and CYLD levels normalized. Together, these findings demonstrate that SPL silencing enhances the severity of IBD and incidence of CAC in a STAT3-dependent manner.
S1P levels are increased in colitic bowel
To explore the role of S1P and SPL in the early stages of IBD independently of CAC, we treated mice with two cycles of DSS without administration of AOM. In these short-term IBD experiments, SPLGutKO mice exhibited more severe IBD disease activity, pathology and STAT3 activation than controls. In both the control and SPLGutKO colon tissues, S1P levels rose in response to DSS treatment, with the latter showing more profound S1P elevation. We next investigated STAT3-target gene expression in colon tissues using a PCR array containing 86 STAT3 transcriptional targets. In SPLGutKO mouse colons, 28 STAT3 targets were upregulated by 2-fold or greater in comparison to the expression levels in control mouse colons. The most significantly affected genes (macrophage inflammatory proteins 1α and 1β, Cd80, Il-2, oncostatin M receptor, genes) are involved in gp130 signaling, proinflammatory, and chemokinetic functions. In addition, miR-21 and miR-181b1 were upregulated in SPLGutKO mouse colons to a greater degree than in control mouse colons in response to DSS. Similar to the findings of others, we observed elevated SphK1 expression in the colon tissues of IBD patients. In addition, we found S1P1 and 66 of 88 other STAT3 target genes to be upregulated in IBD compared to age/gender-matched control colon tissues.
SPL downregulation promotes cell transformation via S1P1/JAK2/STAT3 signaling and microRNA-dependent silencing of the anti-oncogene CYLD
Our studies implicated SPL in regulating CAC at an early step of tumorigenesis, since SPL disruption in gut epithelium led to an increase in tumor incidence but not tumor size. To address the specific role of SPL in the earliest stages of tumorigenesis, we turned to a cellular model. Employing a pair of isogenic mouse embryonic fibroblasts (MEFs) derived from STAT3 KO mice and wild type littermate controls, we silenced SPL in the presence or absence of STAT3. We found that silencing SPL led to an increase in S1P accumulation and transport into the media. S1P export was mediated by the S1P transporter Spns2, as shown by the normalization of extracellular S1P in cells deficient in both SPL and Spn2. In contrast, intracellular S1P levels were unaffected by SPL silencing. This suggests that the cells rapidly export accumulated S1P into the extracellular space, consistent with our finding that Spns2 expression is upregulated in SPL-deficient MEFs. IL-6, the major cytokine inducer of JAK2/STAT3 signaling through the gp130 cell surface receptor, was increased in SPL silenced cells, but only when STAT3 was present. Treatment of MEFs with exogenous S1P led to a modest increase in activation of JAK2 and STAT3, and the activation of both proteins was intensified by the silencing of SPL. Importantly, SPL deficiency led to an increase in the rate of cell proliferation and bestowed MEFs with an ability to form tumors in nude mice, one of the hallmarks of cell transformation. Both of these effects were only observed in the STAT3+ MEF background, demonstrating that SPL silencing induces cell transformation through a STAT3-dependent mechanism. SPL silencing in STAT3+ MEFs also led to an increase in miR-181b1 and reduced expression of its target CYLD. Inhibition of STAT3 signaling, S1P1 signaling or induction of CYLD using chemical modulators reversed the ability of SPL silencing to increase the rate of cell proliferation in STAT3-expressing MEFs. These cumulative findings demonstrate that loss of SPL function and associated extracellular S1P accumulation promote cell transformation through a mechanism involving STAT3 and its targets miR181b1 and S1P1.
Dietary sphingolipids that do not generate S1P protect against IBD and CAC
Our studies demonstrate that S1P accumulation promotes IBD, cell transformation, and CAC. Further, inflammation increases colon S1P levels, likely through upregulation of SphK1 and possibly in response to other changes affecting S1P catabolism. Dietary sphingolipids derived from mammalian food sources, like human sphingolipids, contain a sphingosine backbone and can be converted to S1P in gut epithelium. The inflamed colons of IBD patients, which exhibit SphK1 upregulation, would be adept at converting mammalian sphingolipids to S1P. Further, since they exhibit upregulation of S1PRs, they would be susceptible to S1P signaling and its impact on STAT3 signaling, cytokine induction and CAC.
In contrast, dietary sphingolipids that contain a different structural backbone and cannot be converted to S1P would not be expected to promote inflammation and CAC. We have been studying a class of dietary metabolites called sphingadienes derived from the sphingolipids of soy and other plants (Figure 1) (94). Sphingadienes contain an extra double bond in comparison to the single double bond found in sphingosine (95). Sphingadienes exhibit anti-inflammatory and chemopreventive activities in several models of colon cancer (96,97). We tested the impact of sphingadienes on CAC by delivering them orally to wild type mice concomitant with AOM/DSS. In comparison to vehicle control, we found that sphingadiene administration increased the expression of SPL, CYLD and PTEN in colon tissues (22). In contrast, it reduced colon tissue S1P levels, STAT3 activation and cytokine levels. In addition, in comparison to delivery of sphingosine or a vehicle control, sphingadiene administration cut CAC tumor incidence in half. Together with our other results implicating S1P signaling in IBD and CAC, these findings suggest that dietary sphingolipids may either promote or prevent CAC, depending on their ability to be converted to S1P.
Oxidative stress could modulate SPL activity through post-translational modifications
Oxidative stress is an unavoidable consequence of physiological inflammatory stress (98). Inflammation induced by pathogen exposure or tissue injury increases cellular reactive oxygen and nitrogen species (RONS) through multiple enzymatic sources (99,100). RONS are required for pathogen neutralization, but can also exert deleterious effects by oxidation of macromolecules such as lipids, proteins and DNA (101,102). RONS can also alter protein activities by reversible oxidation of critical thiols of signaling proteins and enzymes (101,102). Interestingly, very little is known regarding the extent to which oxidative stress impacts sphingolipid metabolism through modulation of SPL activity. Considering the important role of SPL in maintaining low S1P levels in gut mucosal cells, changes in SPL activity could have profound effects on inflammation.
Site-directed mutagenesis of human SPL revealed two critical cysteine residues, Cys218 and Cys318, that are required for SPL activity (103). SPL activity is inhibited by the sulfhydryl-alkylating agent, N-ethylmaleimide (NEM) (103). Additionally, inclusion of thiol reducing agents is also required to obtain optimal activities during SPL activity measurements. These observations suggest that: critical cysteine residues are solvent accessible and thus may be susceptible for forming mixed disulfides and for oxidation by RONS and other reactive lipid aldehydes, such as 4-hydroxynonenal and malondialdehyde. It is interesting to note that the human SPL Cys318 residue is flanked by basic amino acids, Lys and Arg (103). Presence of these basic amino acids surrounding Cys residues is likely to decrease the pKa of sulfhydryl moities and increase its susceptibility for redox interactions. During catalysis of S1P cleavage, Cys is required to optimize orientation of PLP and may potentially catalyze retro-aldol cleavage of PLP-S1P intermediate (104-107). Thus, both reversible (e.g., mixed disulfide formation) and irreversible (e.g., oxidation to sulfenic or sulfonic forms) reactions would likely result in SPL inactivation. Additionally, SPL may also interact with the glutaredoxin and thioredoxin family of enzymes that act to reduce reversibly oxidized cysteines. Confirmation of these redox interactions in vivo would provide directly linkage between oxidative stress and SPL and provide mechanisms by which RONS act to enhance inflammation during pathogen invasion and tissue injury.
Nitration of tyrosine by reactive nitrogen species such as peroxynitrite (-ONOO) may be another avenue for oxidative stress dependent modulation of SPL activity. Nitrotyrosine is a hallmark of cellular nitrosative stress (108). Interestingly, Zhan and co-workers discovered evidence for tyrosine nitration on Tyr 355 and Tyr 356 residues (109). Although the consequences of nitrotyrosine formation on SPL activity were not examined, the known role of Tyr in stabilizing PLP in SPL would suggest that nitration would result in inactivation of SPL. Given that SPL activity was found to be decreased in prostate and colon cancer cells (57,58), it may be plausible that nitrotyrosine formation may be an additional mechanism for SPL down-regulation during tumor initiation and progression.
In addition to redox modulation by RONS, SPL may also be subject to phosphorylation-dependent regulation. A survey of potential post-translational modification sites on SPL shows several putative sites for acetylation and phosphorylation (phosphosite.org). Among these, phosphorylation at Ser 564 was confirmed in human cancer, embryonic stem cells, and T lymphocyte cells (110-112). However, these studies did not directly study SPL activity. Detection of phosphorylated SPL in cells undergoing active mitosis and during T-cell receptor activation implicates post-translational modification of SPL as an important regulatory step during mitosis and lymphocyte activation.
Conclusions and future directions
Our studies in murine models and human IBD tissues demonstrate that S1P signaling and metabolism in the gut are influenced by the presence of inflammation. Both SphK1 and S1P1 are upregulated in colitic tissues, and S1P levels are higher in inflamed colon tissues compared to non-inflamed tissues. Other enzymes and receptors involved in S1P metabolism and signaling have not been studied extensively in the context of IBD, and it would be interesting to know whether they also are influenced by the inflammatory milieu. In that context, Sgpl1 gene expression levels may not tell the full story, since deficient vitamin B6 levels in inflamed colons could hamper the enzyme’s activity. Additionally, heightened oxidative stress associated with IBD may further repress SPL activity through post-translational modifications. Considering the high expression of SPL in the differentiated enterocytes of the lower GI tract and the profound effects associated with its loss of function in the colons of SPLGutKO mice, this will be important to examine.
Conversely, it is clear that S1P signaling and metabolism have a profound impact on IBD and CAC. Our findings suggest that S1P signaling and metabolism can modulate the intensity of inflammation and its progression to CAC by increasing STAT3 signaling and its downstream effectors, including microRNAs that have been implicated in cell transformation. The collaborative relationship between STAT3 and S1P signaling suggest that targeting both pathways may be important in reducing CAC incidence in IBD, a condition where both pathways appear to be upregulated and contribute to disease progression.
Recently, investigators reported the discovery of a novel cytokine called PEPITEM that regulates the entry of T lymphocytes into inflamed tissues (113). PEPITEM acts by stimulating S1P production in endothelial cells, leading to S1P release into the blood. T cells adjacent to the endothelium of inflamed tissue receive an S1P-mediated signal which then prevents their ability to undergo transendothelial migration. The PEPITEM pathway represents a self-regulatory mechanism by which the immune system titrates its responses to prevent unbridled inflammation. Importantly, this pathway appears to be dysfunctional in autoimmune diseases such as diabetes and in aging, two conditions in which plasma PEPITEM levels were shown to be reduced. Whether PEPITEM deficiency contributes to pathological inflammation in IBD patients has yet to be tested. If this turns out to be true, the pathway may represent a novel avenue for harnessing S1P-mediated effects to treat IBD and reduce the risk of CAC.
Over the past few years, several cellular processes and molecular factors underlying the development of IBD have been revealed. IBD is caused by dysregulation of homeostatic mechanisms that normally maintain the optimal gut microbiome, intestinal mucosal integrity and function (23,27-43). While many factors are involved in IBD, genetic studies have implicated ER stress, inflammasome activation and autophagy to be key processes that, while designed to coordinately maintain gut cellular homeostasis, can promote inflammation and increase the risk of IBD development when imbalanced (44). ER stress is particularly important in cell types in which protein secretion plays a significant role, such as leukocytes which produce cytokines and gut Paneth cells which are specialized to produce anti-microbial peptides important in barrier maintenance of the gut. ER stress and autophagy have also been implicated in regulating cell death and carcinogenesis, raising the possibility that they are important factors in the progression from IBD to CAC (114,115). Importantly, sphingolipids have been shown to regulate autophagy and have been implicated in ER stress as well (116). Whether S1P signaling and metabolism influence these processes in the context of IBD and CAC remains to be determined. This is an intriguing possibility, considering recent reports by the group of Yoshi Uchida demonstrating that in keratinocytes, ER stress leads to an increase in S1P production and signaling and that both S1P and another sphingolipid ceramide-1-phosphate induce the production of antimicrobial peptides (117-121).
Dietary factors may have a role in the development of IBD and its progression to CAC. Although colon cancer incidence is diminishing in the United States and other westernized countries, this is largely attributed to improved surveillance. In contrast, IBD incidence is rising, and there is evidence to suggest that this is due to dietary changes associated with westernization (122). Our findings have led us to propose a model in which mammalian sphingolipids that can be converted to S1P promote inflammation and CAC, whereas plant sphingolipids that cannot be converted to S1P are anti-inflammatory and chemopreventive. Our finding that SPL is upregulated and STAT3 activation is inhibited in the colon tissues of mice treated with sphingadienes may in part explain their ability to reduce CAC. However, the impact of sphingadienes or other dietary sphingolipids on other aspects of inflammatory signaling has not been tested. Further, the role of endogenous and dietary sphingolipids in other processes implicated in the cause of colon cancer and CAC is unknown. For example, how do sphingolipids influence oxidative stress-induced DNA damage that can lead to induction of procarcinogenic genes and silencing of tumor-suppressor pathways? Do sphingolipids influence the “field change” of cancer-associated molecular alterations (such as p53 mutations, microsatellite instability, epigenetic changes, and additional microRNA alterations) that occur prior to histologic evidence of dysplasia (10,123)? Shifts in bacterial flora and enterotoxigenic species of bacteria have been shown to play a contributory role in CAC and sporadic colon cancer (124). Do dietary sphingolipids alter the gut microbiota, and conversely does the altered microbiota associated with IBD and CAC change the sphingolipid content of the gut and thereby influence epithelial biology, inflammatory signals and carcinogenic stimuli?
A further understanding of how S1P, sphingadienes and other dietary lipids influence the gut mucosa and its innate immune functions will allow the leveraging of sphingolipids to reduce IBD incidence, the extent and severity of inflammation in the disease, and prevent the late consequence of CAC in patients at risk.
Acknowledgments
We would like to thank Teresa Klask for expert administrative assistance. We apologize to those many contributors to this complex field whom we could not cite due to limitations of space.
Funding: This work was supported by Broad Medical Research Program of the Crohn’s and Colitis Foundation of America (CCFA) grant IBD-0353; CCFA Senior Research Award 277014; National Institutes of Health (NIH) grants CA129438 and R21AT005336; American Institute for Cancer Research grant 09A041; and Swim Across America Foundation support (to JD Saba).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hsinyu Lee and Markus H. Gräler) for the series “Lysophospholipids on Immunity and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.10.06). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [PubMed]
- Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med 2010;10:369-73. [PubMed]
- Chai EZ, Siveen KS, Shanmugam MK, et al. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J 2015;468:1-15. [PubMed]
- Thompson PA, Khatami M, Baglole CJ, et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis 2015;36:S232-53. [PubMed]
- Yang B, Kang H, Fung A, et al. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm 2014;2014:623759.
- Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124 [PubMed]
- Crawford S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: a new therapeutic approach to disease progression and recurrence. Ther Adv Med Oncol 2014;6:52-68. [PubMed]
- De Lerma Barbaro A, Perletti G, Bonapace IM, et al. Inflammatory cues acting on the adult intestinal stem cells and the early onset of cancer Int J Oncol 2014;45:959-68. (review). [PubMed]
- Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 2013;35:229-44. [PubMed]
- Aggarwal BB, Sung B. The relationship between inflammation and cancer is analogous to that between fuel and fire. Oncology (Williston Park) 2011;25:414-8. [PubMed]
- Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med 2015;372:1441-52. [PubMed]
- Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol 2005;100:2724-9. [PubMed]
- Kurek K, Łukaszuk B, Piotrowska DM, et al. Metabolism, physiological role, and clinical implications of sphingolipids in gastrointestinal tract. Biomed Res Int 2013;2013:908907.
- Chrisman TD, Perkins DT, Garbers DL. Identification of a potent serum factor that causes desensitization of the receptor for C-Type natriuretic peptide. Cell Commun Signal 2003;1:4. [PubMed]
- Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest 2015;125:1379-87. [PubMed]
- Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355-60. [PubMed]
- Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 2010;9:883-97. [PubMed]
- Bigaud M, Guerini D, Billich A, et al. Second generation S1P pathway modulators: research strategies and clinical developments. Biochim Biophys Acta 2014;1841:745-58.
- Alvarez SE, Harikumar KB, Hait NC, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 2010;465:1084-8. [PubMed]
- Lee H, Deng J, Kujawski M, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 2010;16:1421-8. [PubMed]
- Degagné E, Pandurangan A, Bandhuvula A, et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest 2014;124:5368-84. [PubMed]
- Festen EA, Szperl AM, Weersma RK, et al. Inflammatory bowel disease and celiac disease: overlaps in the pathology and genetics, and their potential drug targets. Endocr Metab Immune Disord Drug Targets 2009;9:199-218. [PubMed]
- Center for Disease Control. Epidemiology of the IBD. 2014. Avaliable online: http://www.cdc.gov/ibd/ibd-epidemiology.htm
- Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol 2003;16:347-58. [PubMed]
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807-16. [PubMed]
- Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics 2009;3:77-97. [PubMed]
- Blumberg RS. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis 2009;27:455-64. [PubMed]
- Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011;140:1729-37. [PubMed]
- Kaser A, Blumberg RS. Adaptive immunity in inflammatory bowel disease: state of the art. Curr Opin Gastroenterol 2008;24:455-61. [PubMed]
- Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res 2012;159:313-25. [PubMed]
- Christophi GP, Rong R, Holtzapple PG, et al. Immune markers and differential signaling networks in ulcerative colitis and Crohn's disease. Inflamm Bowel Dis 2012;18:2342-56. [PubMed]
- Biswas A, Petnicki-Ocwieja T, Kobayashi KS. Nod2: a key regulator linking microbiota to intestinal mucosal immunity. J Mol Med (Berl) 2012;90:15-24. [PubMed]
- Boniface K, Blom B, Liu YJ, et al. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev 2008;226:132-46. [PubMed]
- Dubinsky MC, Wang D, Picornell Y, et al. IL-23 receptor (IL-23R) gene protects against pediatric Crohn's disease. Inflamm Bowel Dis 2007;13:511-5. [PubMed]
- Naser SA, Arce M, Khaja A, et al. Role of ATG16L, NOD2 and IL23R in Crohn's disease pathogenesis. World J Gastroenterol 2012;18:412-24. [PubMed]
- Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol 2008;1:339-49. [PubMed]
- Noble CL, Abbas AR, Lees CW, et al. Characterization of intestinal gene expression profiles in Crohn's disease by genome-wide microarray analysis. Inflamm Bowel Dis 2010;16:1717-28. [PubMed]
- Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009;15:103-13. [PubMed]
- Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol 2010;38:76-87. [PubMed]
- Hayakawa Y, Maeda S, Nakagawa H, et al. Effectiveness of IkappaB kinase inhibitors in murine colitis-associated tumorigenesis. J Gastroenterol 2009;44:935-43. [PubMed]
- Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119-24. [PubMed]
- Wallace KL, Zheng LB, Kanazawa Y, et al. Immunopathology of inflammatory bowel disease. World J Gastroenterol 2014;20:6-21. [PubMed]
- Latella G, Fiocchi C, Caprili R. News from the "5th International Meeting on Inflammatory Bowel Diseases" CAPRI 2010. J Crohns Colitis 2010;4:690-702. [PubMed]
- Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut 2011;60:1739-53. [PubMed]
- McGovern DP, Gardet A, Torkvist L, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 2010;42:332-7. [PubMed]
- Breslow DK, Collins SR, Bodenmiller B, et al. Orm family proteins mediate sphingolipid homeostasis. Nature 2010;463:1048-53. [PubMed]
- Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog Lipid Res 2009;48:62-72. [PubMed]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008;9:139-50. [PubMed]
- Furuya H, Shimizu Y, Kawamori T. Sphingolipids in cancer. Cancer Metastasis Rev 2011;30:567-76. [PubMed]
- Pyne S, Lee SC, Long J, et al. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine-1-phosphate signalling in health and disease. Cell Signal 2009;21:14-21. [PubMed]
- Serra M, Saba JD. Sphingosine-1-phosphate lyase, a key regulator of sphingosine-1-phosphate signaling and function. Adv Enzyme Regul 2010;50:349-62. [PubMed]
- Vogel P, Donoviel MS, Read R, et al. Incomplete inhibition of sphingosine-1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One 2009;4:e4112 [PubMed]
- Siow DL, Anderson CD, Berdyshev EV, et al. Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J Lipid Res 2010;51:2546-59. [PubMed]
- Kohno M, Momoi M, Oo M, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol 2006;26:7211-23. [PubMed]
- Kawamori T, Kaneshiro T, Okumura M, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J 2009;23:405-14. [PubMed]
- Oskouian B, Sooriyakumaran P, Borowsky A, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is downregulated in colon cancer. Proc Natl Acad Sci U S A 2006;103:17384-9. [PubMed]
- Brizuela L, Ader I, Mazerolles C, et al. First evidence of sphingosine-1-phosphate lyase protein expression and activity downregulation in human neoplasm: implication for resistance to therapeutics in prostate cancer. Mol Cancer Ther 2012;11:1841-51. [PubMed]
- Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine-1-phosphate receptors. J Biol Chem 2002;277:21453-7. [PubMed]
- Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002;296:346-9. [PubMed]
- Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine-1-phosphate G-protein-coupled receptors. FASEB J 2004;18:551-3. [PubMed]
- Oo ML, Thangada S, Wu MT, et al. Immunosuppressive and anti-angiogenic sphingosine-1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem 2007;282:9082-9. [PubMed]
- Quancard J, Bollbuck B, Janser P, et al. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem Biol 2012;19:1142-51. [PubMed]
- Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005;309:1735-9. [PubMed]
- Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 2010;328:1129-35. [PubMed]
- Sensken SC, Bode C, Nagarajan M, et al. Redistribution of sphingosine-1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J Immunol 2010;184:4133-42. [PubMed]
- Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355:1124-40. [PubMed]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 2008;8:753-63. [PubMed]
- Kawamori T, Osta W, Johnson KR, et al. Sphingosine kinase 1 is upregulated in colon carcinogenesis. FASEB J 2006;20:386-8. [PubMed]
- Liang J, Nagahashi M, Kim EY, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013;23:107-20. [PubMed]
- Borowsky AD, Bandhuvula P, Kumar A, et al. Sphingosine-1-phosphate lyase expression in embryonic and adult murine tissues. J Lipid Res 2012;53:1920-31. [PubMed]
- Maines LW, Fitzpatrick LR, French KJ, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci 2008;53:997-1012. [PubMed]
- Snider AJ, Kawamori T, Bradshaw SG, et al. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J 2009;23:143-52. [PubMed]
- Chumanevich AA, Poudyal D, Cui X, et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 2010;31:1787-93. [PubMed]
- Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol 2013;718:145-53. [PubMed]
- Selhub J, Byun A, Liu Z, et al. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem 2013;24:2138-43. [PubMed]
- Paul L, Ueland PM, Selhub J. Mechanistic perspective on the relationship between pyridoxal 5'-phosphate and inflammation. Nutr Rev 2013;71:239-44. [PubMed]
- Degagné E, Saba JD. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin Exp Gastroenterol 2014;7:205-14. [PubMed]
- Daniel C, Sartory N, Zahn N, et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol 2007;178:2458-68. [PubMed]
- Fujii R, Kanai T, Nemoto Y, et al. FTY720 suppresses CD4+CD44highCD62L- effector memory T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol 2006;291:G267-74. [PubMed]
- Deguchi Y, Andoh A, Yagi Y, et al. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol Rep 2006;16:699-703. [PubMed]
- Mizushima T, Ito T, Kishi D, et al. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis 2004;10:182-92. [PubMed]
- Sanada Y, Mizushima T, Kai Y, et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One 2011;6:e23933 [PubMed]
- Song J, Matsuda C, Kai Y, et al. A novel sphingosine-1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther 2008;324:276-83. [PubMed]
- Schmahl J, Raymond CS, Soriano P. PDGF signaling specificity is mediated through multiple immediate early genes. Nat Genet 2007;39:52-60. [PubMed]
- Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature 2007;446:676-9. [PubMed]
- Kusaba T, Nakayama T, Yamazumi K, et al. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep 2006;15:1445-51. [PubMed]
- Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 2005;7:545-55. [PubMed]
- Lin Q, Lai R, Chirieac LR, et al. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol 2005;167:969-80. [PubMed]
- Leslie K, Gao SP, Berishaj M, et al. Differential interleukin-6/Stat3 signaling as a function of cellular context mediates Ras-induced transformation. Breast Cancer Res 2010;12:R80. [PubMed]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009;15:79-80. [PubMed]
- Wang Z, Jin H, Xu R, et al. Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Exp Mol Med 2009;41:717-27. [PubMed]
- Iliopoulos D, Jaeger SA, Hirsch HA, et al. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 2010;39:493-506. [PubMed]
- Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol 2010;6:489-97. [PubMed]
- Fyrst H, Zhang X, Herr DR, et al. Identification and characterization by electrospray mass spectrometry of endogenous Drosophila sphingadienes. J Lipid Res 2008;49:597-606. [PubMed]
- Kumar A, Pandurangan AK, Lu F, et al. Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3β pathway in colon cancer. Carcinogenesis 2012;33:1726-35. [PubMed]
- Fyrst H, Oskouian B, Bandhuvula P, et al. Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res 2009;69:9457-64. [PubMed]
- Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603-16. [PubMed]
- Suh JH, Kim RY, Lee DS. A new metabolomic assay to examine inflammation and redox pathways following LPS challenge. J Inflamm (Lond) 2012;9:37. [PubMed]
- Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease--radicals or ridiculous? Aliment Pharmacol Ther 2002;16:1997-2015. [PubMed]
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 2015;4:180-3. [PubMed]
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 2007;35:1147-50. [PubMed]
- Van Veldhoven PP, Gijsbers S, Mannaerts GP, et al. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochim Biophys Acta 2000;1487:128-34. [PubMed]
- Bourquin F, Capitani G, Grütter MG. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci 2011;20:1492-508. [PubMed]
- Bourquin F, Riezman H, Capitani G, et al. Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism. Structure 2010;18:1054-65. [PubMed]
- Copley SD, Novak WR, Babbitt PC. Divergence of function in the thioredoxin fold suprafamily: evidence for evolution of peroxiredoxins from a thioredoxin-like ancestor. Biochemistry 2004;43:13981-95. [PubMed]
- Szajewski RP, Whitesides GM. Rate constants and equilibrium constants for thiol-disulfide interchange reactions involving oxidized glutathione. J Am Chem Soc 1980;102:2011-26.
- Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys 1998;356:1-11. [PubMed]
- Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal Biochem 2006;354:279-89. [PubMed]
- Mayya V, Lundgren DH, Hwang SI, et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal 2009;2:ra46. [PubMed]
- Kim JY, Welsh EA, Oguz U, et al. Dissection of TBK1 signaling via phosphoproteomics in lung cancer cells. Proc Natl Acad Sci U S A 2013;110:12414-9. [PubMed]
- Kettenbach AN, Schweppe DK, Faherty BK, et al. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal 2011;4:rs5. [PubMed]
- Chimen M, McGettrick HM, Apta B, et al. Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat Med 2015;21:467-75. [PubMed]
- Lu M, Lawrence DA, Marsters S, et al. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 2014;345:98-101. [PubMed]
- Verfaillie T, Salazar M, Velasco G, et al. Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy. Int J Cell Biol 2010;2010:930509.
- Ponnusamy S, Meyers-Needham M, Senkal CE, et al. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol 2010;6:1603-24. [PubMed]
- Park K, Elias PM, Shin KO, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol 2013;33:752-62. [PubMed]
- Park K, Elias PM, Hupe M, et al. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol 2013;133:1942-9. [PubMed]
- Park K, Kim YI, Shin KO, et al. The dietary ingredient, genistein, stimulates cathelicidin antimicrobial peptide expression through a novel S1P-dependent mechanism. J Nutr Biochem 2014;25:734-40. [PubMed]
- Kim YI, Park K, Kim JY, et al. An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating beta-defensin production. Mol Cell Biol 2014;34:4368-78. [PubMed]
- Jeong SK, Kim YI, Shin KO, et al. Sphingosine kinase 1 activation enhances epidermal innate immunity through sphingosine-1-phosphate stimulation of cathelicidin production. J Dermatol Sci 2015; [Epub ahead of print]. [PubMed]
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42; quiz e30.
- Dotto GP. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest 2014;124:1446-53. [PubMed]
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014;12:661-72. [PubMed]


