
Lysophospholipid receptor signaling in zebrafish development
Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are membrane-derived lysophospholipids important for their functions in different physiological and patho-physiological conditions (1). LPA is synthesized mainly from lysophosphatidylcholine by autotaxin with a phospholipase D (lysoPLD) activity (2,3) and act via cognate G-protein-coupled receptors (GPCRs) to exert their effects via remodeling actin cytoskeleton or altering gene expression (1). S1P can be generated via phosphorylation of sphingosine by sphingosine kinases and its S1P level can be modulated by additional enzymes like sphingosine phosphatases, and sphingosine lyase (4,5). S1P was initially found to be a sphingolipid that elevates intracellular calcium and cell proliferation (6). Similar to LPA receptors, S1P receptors also function via cognate GPCRs (7,8). Here, the synthesis and receptor signaling will be described for both LPA and S1P. We will also discuss their roles in vivo with an emphasis on those recently accumulated zebrafish data.
Autotaxin (ATX)
ATX, also named ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), is one of seven members in the ENPP family. They hydrolyze nucleotides in vitro and are classified as ecto- and exo-enzymes (9). ATX is a 125 KDa glycoprotein first isolated in a conditioned medium of A2058 melanoma cells. It is a secreted protein with a high cancer cell motility stimulating activity that is pertussis toxin (PTX)-sensitive and thus should be mediated via receptor(s) coupled to PTX—dependent G protein (10). ATX increases tube formation in matrigel assays (11-13) and enhances metastasis and angiogenesis (13). It was hypothesized that ATX may provide microenvironments favoring invasion and/or angiogenesis for malignant cells to promote tumor progression. However, the biochemical activity of ATX was not known until it was found to contain lysoPLD activity that can hydrolyze lysophosphatidylcholine into LPA (3,14).
ATX is initially synthesized as a pre-pro-enzyme. Its N-terminal signal peptide is first removed and then followed by its cleavage using convertases and secretion into the extracellular space where it exerts lysoPLD activity (15-17). An active ATX contains two N-terminal somatomedin B-like (SMB) domains, a central catalytic phosphodiester domain (PDE) and a C-terminal nuclease-like (NUC) domain. The PDE domain has a lipid binding pocket and a flanking tunnel that may serve as a lysophospholipid entry and/or exit site (18). The NUC domain helps the rigidity of the PDE domain, while the SMB domains assist in the binding of ATX to integrins (19) that brings ATX to the vicinity of LPA cognate receptors and then those receptors can be activated by LPA synthesized by ATX (18,20,21). Although the biochemical and structural properties ATX have been intensively studied, the regulatory mechanisms of ATX activity and the control of LPA release are still illusive. Intriguingly, despite their similarity between catalytic domains, ATX is the only ENPP family member containing intrinsic lysoPLD activity. But LPA can also be synthesized by other ecto/exo-phospholipases, including phosphatidic acid (PA)-specific phospholipase A1 (22) and sphingomyelinases D (23).
LPA and LPA receptors
Lysophospholipids are minor membrane components and also extracellular signaling molecules found in many tissues and biological fluids. LPA is one of the major lysophospholipids (24). Diverse LPA activities have been reported in different cellular and developmental studies. LPA can affect cell survival, proliferation, migration, adhesion, morphology and other cellular functions of various cell types via GPCR-mediated signaling (25,26). Cell types affected include neuronal cells like neural progenitor cells, astrocytes and oligodendrocytes (27), vascular endothelial cells (28), osteoblasts and osteoclasts (29,30) proliferating pre-adipocytes (31), reproductive-related cells (32) and immune cells (33,34).
LPA is a metabolite derived from the synthesis of membrane phospholipids. It is ubiquitously present in many tissues examined. LPAs have various forms with different acyl chain lengths, saturation, and position. The most commonly used LPA is 18:1 oleoyl-LPA (1-acyl-2-hydroxy-sn-glycero-3-phosphate). LPA is present in significant amount in blood fractions from 0.1 µM to more than 10 µM in plasma and serum, respectively (35,36). LPA concentrations at those levels are higher than the Kd values of LPA receptors for LPA, and thus should have enough LPA to exert their functions.
LPA receptors exert their effect via remodeling actin cytoskeleton or altering gene expression (37). At least six GPCRs for LPA, named LPA1-LPA6 (or LPAR1-LPAR6), have been identified. Similar to S1P receptors, but only three of those LPA receptors (LPA1-3) belong to the endothelial-derived growth factor (Edg) receptor family (38,39). LPA receptors are more closely related to the purinergic GPCRs (38-41). They are widely expressed with overlapping and distinct signaling and tissue distributions (38,40).
ATX–LPA signaling in zebrafish
The so called “ATX-LPA signaling axis” (13) is implicated in a variety of physiological and patho-physiological processes, including vascular and neural development (13,42-45), tumor progression and metastasis (46,47), lymphocyte trafficking (21), bone development (48), neuropathic pain (49), pulmonary fibrosis (50), fat mass regulation (51), cholestatic pruritus (52), fetal hydrocephalus (53) and chronic inflammation (54). Those studies were mainly carried out in mammals. Not until recently, LPA and its close relative S1P have attracted intensive research in zebrafish.
Zebrafish as a vertebrate model
Zebrafish is a versatile vertebrate model to investigate early embryogenesis and organogenesis including vascular and neuronal development. The major advantages of using zebrafish as a model are as following: (I) excellent embryology: external fertilization, rapid development and optical transparency allow easy observation and manipulation; (II) small size (about an inch in length) and relative short generation (3 months): allow genetic study in large quantity; (III) accessibility of genome manipulation: tools available for transgenesis and mutagenesis. Zebrafish is particularly suitable for cardiovascular studies because the optical clarity and their embryos can develop in the absence of blood circulation within 7 days post fertilization. These characteristics make zebrafish a superior model than mammalian species. In fact, many novel discoveries have been made in zebrafish for our understanding in vascular development (55).
Vascular development in zebrafish
Vertebrate vasculature is mainly composed of two systems, blood and lymphatic vasculatures, that develop in parallel. Blood vessels mediate the transportation and exchange of gas, nutrients and metabolites. Lymphatic vessels mainly function to regulate immunity via immune cells and to absorb gut lipids and body fluids. Endothelial cells shape the most interior layer of both blood and lymphatic vessels. Two waves of angiogenic sprouting occur in zebrafish (56). Blood endothelial cells (BECs) of the primary wave sprout from the dorsal aorta (DA) at 22 hours post fertilization (hpf) to form the segmental arteries (SA). These BECs grow dorsally, reach the dorsal neural tube and connect with their neighbors from anterior and posterior segments to form the dorsal longitudinal anastomotic vessel (DLAV). The second wave is the development of lymphatic endothelial cells (LECs), which come from the posterior cardinal vein (PCV) to form lymphatic vessel (57).
Lymphangiogenesis is a process of the formation of new lymphatic vessels from pre-existing lymphatics (58). In zebrafish, lymphatic vessel development starts at 32 hpf (57). The lymphatic vessels are distributed into four regions: facial lymphatic network, intestinal lymphatic network, lateral lymphatic network and trunk lymphatic network (59). The trunk lymphatic network, which consists of thoracic duct, intersomitic lymphatic vessels (ISLVs) and dorsal longitudinal lymphatic vessels (DLLVs), has been most widely studied (60). Among trunk lymphatic vessels, the thoracic duct has often been used as a model to study lymphangiogenesis because of its big size and better visibility (60,61). The thoracic duct is connected to an existing SA and is transformed into segmental veins (SV). They can also reach the horizontal myoseptum (HM) to form parachordal lymphangioblasts (PLs). Between 60 and 84 hpf, PLs migrate ventrally to give rise to the ventral part of the ISLVs or dorsally to form the DLLVs (57,61). The majority of PLs eventually migrate away from the horizontal myoseptum and contribute to the lymphatic vasculature (61).
Lymphangiogenesis is balanced between pro- and anti-lymphangiogenic factors to maintain its homeostasis. VEGF, HGF and FGF2 signaling are well known pro-lymphangiogenic factors (62). They affect collagen and calcium binding EGF domains 1 (CCBE 1), vascular endothelial growth factor C (VEGFC), prospero-related homeobox gene 1 (PROX1), and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) to regulate lymphangiogenesis (63). VEGFC and its receptor VEGFR3 play a key role during multiple developmental stages of lymphatic vessel. The sprouting of LECs from veins is VEGFC-dependent. VEGFR3 receptors are expressed in endothelial cells, including in PROX1-positive lymphatic precursors and blood vessel precursors (64). The VEGFR3 co-receptor neuropilin 2 (NRP2) modulates the signaling pathways that are activated in response to VEGFC and VEGFD (65). PROX1 is the major regulator of LECs fate and the most reliable marker of LEC identity (66). Its activity is crucial for the appearance of LEC progenitors to leave the cardinal vein during lymphatic development (67,68). PROX1 and its regulators COUP-TFII and SOX18 drive LECs specification in mice. The cooperative control of early LEC fate induction is intriguing during this process (69). LYVE1 is one of the most specific and commonly used mammalian lymphatic endothelial markers. A LYVE1 orthologue has also been found in zebrafish (70,71).
ATX-LPA signaling in vascular development
The role of ATX in vascular development has been well documented in mice. At embryonic day 9.5 (E9.5), ATX-knockout mice die and suffer with sever yolk sac vascular defects and other defects. The appearance of abnormalities matches the timing of elevated expression of ATX and LPA receptors in wild-type embryos (45). Since ATX is the main synthesizing enzyme for LPA, it suggests that the vascular abnormality in ATX-knockout embryos may be caused by deficiency of LPA signaling through LPA receptors. However, the loss of function assay of LPA receptors initially failed to demonstrate their importance in vascular development. Mice lacking LPA1, LPA2 or LPA3 do not show notable vascular defects except hemorrhage in the frontal head of LPA1 or LPA1/2 double-knockout mice. Neonatal LPA1-deficient mice show abnormal suckling behavior presumably due to defects in olfactory system. LPA2-knockout mice appear normal. LPA1/2 double-knockout mice show no additional defects but a higher incidence (26%) of frontal hematoma was observed in the neonatal mice as compared to that (2.5%) of LPA1 knockout mice (72). LPA3-deficiency results in abnormal embryonic spacing in uterus and impaired implantation in mice (32). Collectively, those studies in mice do not support a role of LPA receptors in mediating vascular development in vivo, therefore how ATX/LPA mediates vascular development in vivo remains unresolved.
LPA receptor signaling in lymphangiogenesis
To clarify the unresolved issue, we decided to further study the in vivo functions of LPA receptors in zebrafish because its advantages to study vascular development in vivo. We have cloned and analyzed expression patterns of lpa1-lpa3 genes in zebrafish (73,74). The LPA1-LPA3 proteins are highly homologous to their mammalian homologues with at least 60% identity. We knocked down lpa1 by antisense morpholino oligonucleotides (MO) and found that the development of early blood vasculature is only mildly delayed at the dosages tested. However, edema gradually appears in pericardium and mid-trunk regions. Further examination revealed that the formation of the major lymphatic vessel, thoracic duct, is inhibited and thus tissue fluid cannot be drained via lymphatic vessels (74). This is the first in vivo evidence to document the importance of any LPA receptors in vascular development. Mechanistically, LPA1 may act via its regulation on VEGFC, one of the key regulators of lymphatic vessels (75). We demonstrated that VEGFC rescues lymphatic defects in lpa1-deficient embryos. It suggested that LPA1 exerts its effects on lymphangiogenesis via VEGFC. To explore the link between LPA and VEGFC signaling, Lin et al. showed that LPA stimulates the expression of VEGFC and other lymphatic marker genes, including PROX-1, LYVE-1 and Podoplanin, and tube formation in human umbilical vein endothelial cells (HUVECs). Furthermore, these LPA-mediated events depend on LPA1/LPA3 signaling and EGF transactivation to activate NF-kB mediated VEGFC expression (76,77). HUVECs are blood vessel endothelial cells that may not fully support a role of LPA in lymphangiogenesis. Using human lymphatic endothelial cells LPA was shown to induce lymphangiogenesis and IL-8 production in vitro (78). More interestingly, LPA was shown to enhance VEGFC expression in human prostate cancer PC-3 cells. It implied that LPA-dependent VEGFC expression and lymphangiogenesis may be involved in cancer metastasis (79).
The lack of notable vascular phenotype in LPA1-LPA3-deficient mice is in clear contrast to that of zebrafish embryos. One possibility is that different or multiple LPA receptors are involved. Indeed, it may be the case. Less than 20% of LPA4-deficient mouse embryos died during gestation showing hemorrhages and/or edema in various organs at different stages. Both blood and lymphatic vessel were often dilated that might be attributed to defects in recruitment of mural cells and/or pericytes (80). Taken together, the ATX/LPA axis is critical in the establishment of vasculature via different LPA receptors in different species.
Biochemical properties of zebrafish ATX and LPA have been comprehensively characterized (81). Similar to its mammalian homologues, zebrafish ATX also contains lysophospholipase D activity to produce LPA. We also observed similar results (73). All zebrafish LPA receptors were shown to react to LPA but not LPA5a and LPA5b. The ATX-MO causes abnormal vascular branching that is similar to that observed in the ATX-null mice (45) and a hampered blood circulation in zebrafish embryos (Table 1). Zebrafish embryos injected with MOs against individual LPA receptors have normal vasculature. In contrast, intersegmental arteries sprout normally from the dorsal aorta but stop at horizontal myotectum with aberrant vascular connection in a portion of embryos treated with MOs against both LPA1 and LPA4 (81). These results further demonstrate the necessity of ATX-LPA-LPAR axis for proper vascular development.

Full table
ATX-LPA signaling in left-right asymmetry
The abnormal vasculature and circulation are caused by severe stretching of atria to ventricle and weak heart contraction in atx morphants. Interestingly, cardiac jogging and looping were often observed in those morphants which is a sign of an interference of establishing left–right (L-R) patterning (82). The L-R axis is one of the body axes that are fundamental to embryogenesis. L-R asymmetry occurs in vertebrates at different stages and has been studied extensively. The node is a key embryonic structure, which is essential for establishing L-R patterning. The equivalent structure of the node is the Kupffer’s vesicle (KV) in zebrafish (83,84). The midline, mainly consisting of the floor plate and notochord, is a barrier which expresses lefty1to prevent signals from intermingling between left and right (85). Dorsal forerunner cells (DFCs), a group of non-involuting cells at the front of the dorsal shield, are precursors of KV (86-88). DFCs first form a rosette-like structure and then a cavity is created within it to become a vesicle. Cilia are inside the lumen of vesicles and asymmetrically placed along the anterior-posterior axis. The beating of cilia generates a counter-clockwise fluid movement called nodal flow. The nodal flow then drives the asymmetrical expression of genes, such as nodal-related southpaw (spaw), pitx2 and lefty, in the left lateral plate mesoderm to direct the laterality of organogenesis (83,89,90).
The formation of KV or node appears to be one of the earliest signals for establishing L-R asymmetry. However, the mechanism regulating KV formation is largely unknown until recently. At the mid-epiboly, the dorsal shield has an increased intracellular calcium level that was demonstrated to be essential for the collective migration of DFCs and formation of KV in the tail bud later on. Perturbing calcium signaling using thapsigargin, a calcium ATPase inhibitor, blocks KV formation and disrupts subsequent L-R patterning (91). However, the mechanism regulating the calcium elevation at the shield remained unclear at that time. The defects in cardiac jogging and looping observed in atx or lpa3 MO-injected embryos might be due to the early perturbation of L-R patterning. Furthermore, one of the LPA downstream signals is the activation of phospholipase C and elevation of intracellular calcium (25). So we reasoned that LPA is the key trigger of the dorsal shield calcium. Using antisense MO, we showed that the blockade of ATX-LPA3 receptor axis inhibits the formation of KV, and later the expression of asymmetric genes, lefty1/2, pitx2 and spaw. The dorsalized shield calcium may be induced by LPA synthesized by the enriched ATX therein. It is also associated with Wnt signaling, as shown by the accumulation of β-catenin at the dorsal nuclei of both atx and lpa3 morphants (60).
LPA receptors in neuronal development
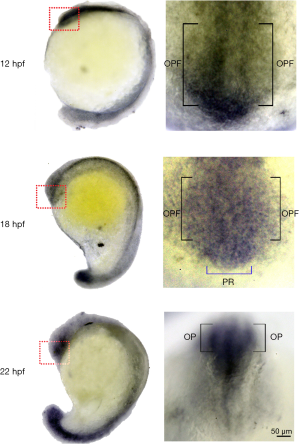
The roles of LPARs in neural development have been suggested by the initial discovery of the enrichment of LPAR1 in the brain ventricular that earned its original name, ventricular zone gene-1 (VZG1) (92). Different LPAR subtypes are expressed at different stage and cell types in the developing and mature cerebral cortex. They are also expressed in ependyma, blood-brain barrier, and meninges. The LPARs expression patterns change while progenitors appear in the ventricular zone (VZ) and differentiate during their migration to the subventricular zone (SVZ) and intermediate zone (IZ), and then situate in cortical plate (CP). During the formation of embryonic brain, LPA mediates proliferation, interkinetic nuclear migration, cell migration, neurite retraction and survival. After birth, LPA signaling regulates neuron myelination, responses of microglial and astrocytic, vascular integrity, and cognition (93). Not much has been done to examine the role of LPA signaling in zebrafish neuronal development. Our preliminary data revealed that the formation of brain ventricle is impaired in ATX morphants. In addition, we found that lpa1 is expressed in the olfactory pre-placodal region and olfactory placodal field (OPF) of developing zebrafish embryos (Figure 1). This is intriguing because the postnatal LPA1 null mice die because of the difficulty of milk suckling and presumably harboring an impaired olfaction (94). To examine this notion, we tested the olfactory response of lpar1 morphants and observed that they have reduced response to the L-alanine, an appetitive odorant. However, how the loss of LPA1 hampers the olfaction in zebrafish remains to be elucidated.
S1P and S1P receptors
S1P is produced via the metabolism of sphingomyelin on cell membrane. Sphingomyelin is hydrolyzed by sphingomyelinase to form phosphocholine and ceramide. Ceramide is then catalyzed by ceramidase to become sphingosine, an immediate precursor of S1P. Sphingosine is then phosphorylated by sphingosine kinases to generate S1P (4,5). S1P is found in all cells examined, however, erythrocytes and endothelial cells are in general considered to be the major sources of S1P in plasma under normal physiology (95). The homeostasis of S1P is regulated by several enzymes, including sphingosine kinases, lysophospholipid phosphatases, and S1P lyase (4,5). S1P like LPA is secreted extracellularly for its actions. The secretion of S1P to the extracellular space is via transporters like ATP-binding cassette (ABC)-type transporters, ABCC1, ABCA1 and ABCG1Jeny (96-98). The regulatory mechanism for S1P transportation via the ABC transporters is still unclear. But recent works in zebrafish have revealed that the Spns2 gene encodes a specific S1P transporter (99,100). Upon entering circulation, S1P is associated with high-density lipoproteins and other plasma proteins like albumin to provide a stable reservoir of S1P for its subsequent activation of five known S1P receptors (S1PRs, S1P1-S1P5) (101).
S1P receptors have distinct but overlapping intracellular signaling cascades (102). S1P1-S1P3 are expressed in different tissues, including the central nervous system (CNS), immune cells, heart and vasculature (103). S1P4 is not only found in spleen CD4 and CD8 T cells (104) but in cells of the hematopoietic and lymphoid lineages. S1P5 is expressed on natural killer cells and oligodendrocytes (105). S1PRs mainly function in the immune, cardiovascular and CNS systems.
Lymphocytes circulate between the blood and lymphatic systems that is essential for immune function. It is known that the S1P-S1P1 signal is critical for the circulation of naïve B and T lymphocytes. Lymphatic vascular cells expressing lymphatic vessel endothelium receptor-1 can secrete S1P to activate S1P1 on lymphocytes. This results in the exit of lymphocytes from lymph nodes and concomitant transient downregulation of lymphocyte expression of S1P1 such that the lymphocytes fail to be activated by S1P and thus remain in lymph nodes. Activation of lymphocytes and following clonal expansion allows S1P1 to be is re-expressed on lymphocytes and regain its responsiveness to S1P and ability of egression from lymph nodes (95,106-108).
S1PRs are expressed on the membranes of neurons, astrocytes, oligodendrocytes, and microglial cells in the CNS (109,110). However, the roles of S1PRs for the CNS are just beginning to be understood. In vitro and animal models studies revealed that S1P is critical for conditions like migration of neuronal progenitor cells toward injured areas; astrocyte migration and communication with other CNS cells; oligodendrocyte survival, myelination following injury; regulation of microglial number and activation; and the integrity of the blood-brain barrier (40,111-119).
S1P1 and S1P3 collaboratively induce an acute but transient decrease in heart beats (120,121). S1P1 is highly expressed in cardiomyocytes of ventricles, septa, and atria and in endothelial cells of cardiac vessels (122). S1P1 but not S1P3 is the major regulator of atrial myocyte contraction and heart rate. Collectively, it suggests that S1P may be involved in the regulation of heart rate, but the relative importance of S1P1 and S1P3 may depend on experimental models and animal species (123).
Smooth muscle cells of S1P1-null mice develop nascent endothelial tubes. However, the process of vascular maturation is disrupted (124,125). In vivo and in vitro human studies in conjunction with animal studies suggest that the S1P1palys a significant role in guiding vascular development (126). In addition, the S1P2 receptor has also be implicated in atherosclerosis (127).
Genetic ablation revealed essential roles of S1prs during development in mice (128). S1pr1-knockout mice have severe hemorrhage and die between E12.5 and E14.5 (129), and S1pr1, S1pr2 and S1pr3-triple mutants have more profound vascular phenotypes (130). It implies a pivotal role of S1pr1 together with S1pr2 and S1pr3 in vascular development in mice.
S1P signaling in zebrafish
Sphingosine kinases (SPHK1 and SPHK2) are S1P synthesizing enzymes by phosphorylating sphingosine. But their roles in vivo have been obscure until recently. Two independent studies in zebrafish generated transcription activator-like effector nuclease (TALEN)-targeted mutations in sphk1 and sphk2 (131,132). Both groups found that the sphk2 zygotic mutants grow normally to adulthood, but the sphk2 maternal-zygotic mutant showed cardiac bifida phenotypes as that observed in the mutants carrying S1P transporter spns2 or S1P receptor s1pr2 mutations (99,100,133). In contrast, no cardiac defect was observed in sphk1 maternal-zygotic mutants (132). These results clearly demonstrated that the Sphk2-Spns2-S1pr2 axis mediates the cardiac progenitor migration in zebrafish through maternal and zygotic regulation.
Seven S1PRs have been identified and isolated in zebrafish. Comprehensive gene expression and functional analyses had been done for those zebrafish S1PRs (134). Those s1prs have unique and overlapping expression domains during early embryogenesis. Using TALEN-mediated mutagenesis, all s1pr mutant zebrafish had been generated and analyzed for their phenotypes during early embryogenesis. S1pr2 mutant show cardiac bifida phenotype that is consistent with a previous identified mutants against the same gene (133,135). Both zygotic and maternal zygotic mutants for other s1prs normally develop to adulthood. It can be occurrence of gene compensation in these deleterious mutations (136) or gene redundancy among S1PRs. The later has been suggested by preliminary data reporting early embryonic defects including vasculogenesis in s1pr3b and s1pr4-double mutants (134).
Concluding remarks
Despite of comprehensive analyses of ATX and receptors for LPA and S1P in mice, their studies in zebrafish are just beginning to be unraveled. Currently, many functions appear to be conserved in zebrafish. With the ease in gene functional analysis in zebrafish, it provides a great platform for further studies for LPA and S1P signaling. In particular, the feasibility of targeted genome editing like TALEN and clustered, regularly interspaced, short palindromic repeats (CRISPR) (137) technologies in zebrafish provides us more critical tools and strengthen our ability to understand the field of lysophospholipid signaling.
Acknowledgments
Funding: The work was supported by the Ministry of Science and Technology (MOST103-2321-B-002-009-) and National Taiwan University (NTU CESRP-10R70602A5 and NTU ERP-10R80600) to SJL.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hsinyu Lee and Markus H. Gräler) for the series “Lysophospholipids on Immunity and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.10.03). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol 2008;160:25-46. [PubMed]
- Fukushima N, Weiner JA, Kaushal D, et al. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol Cell Neurosci 2002;20:271-82. [PubMed]
- Umezu-Goto M, Kishi Y, Taira A, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 2002;158:227-33. [PubMed]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol 2004;15:513-20. [PubMed]
- Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011;76:S3-8. [PubMed]
- Zhang H, Desai NN, Olivera A, et al. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol 1991;114:155-67. [PubMed]
- Spiegel S. Sphingosine 1-phosphate: a ligand for the EDG-1 family of G-protein-coupled receptors. Ann N Y Acad Sci 2000;905:54-60. [PubMed]
- Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem 2002;131:767-71. [PubMed]
- Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci 2005;30:542-50. [PubMed]
- Stracke ML, Krutzsch HC, Unsworth EJ, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 1992;267:2524-9. [PubMed]
- Nam SW, Clair T, Campo CK, et al. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene 2000;19:241-7. [PubMed]
- Nam SW, Clair T, Kim YS, et al. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res 2001;61:6938-44. [PubMed]
- van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res 2007;46:145-60. [PubMed]
- Tokumura A, Majima E, Kariya Y, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 2002;277:39436-42. [PubMed]
- Jansen S, Stefan C, Creemers JW, et al. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J Cell Sci 2005;118:3081-9. [PubMed]
- Koike S, Keino-Masu K, Ohto T, et al. The N-terminal hydrophobic sequence of autotaxin (ENPP2) functions as a signal peptide. Genes Cells 2006;11:133-42. [PubMed]
- van Meeteren LA, Ruurs P, Christodoulou E, et al. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem 2005;280:21155-61. [PubMed]
- Moolenaar WH, Perrakis A. Insights into autotaxin: how to produce and present a lipid mediator. Nat Rev Mol Cell Biol 2011;12:674-9. [PubMed]
- Hausmann J, Kamtekar S, Christodoulou E, et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol 2011;18:198-204. [PubMed]
- Fulkerson Z, Wu T, Sunkara M, et al. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem 2011;286:34654-63. [PubMed]
- Kanda H, Newton R, Klein R, et al. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol 2008;9:415-23. [PubMed]
- Inoue A, Arima N, Ishiguro J, et al. LPA-producing enzyme PA-PLA1α regulates hair follicle development by modulating EGFR signalling. EMBO J 2011;30:4248-60. [PubMed]
- van Meeteren LA, Frederiks F, Giepmans BN, et al. Spider and bacterial sphingomyelinases D target cellular lysophosphatidic acid receptors by hydrolyzing lysophosphatidylcholine. J Biol Chem 2004;279:10833-6. [PubMed]
- Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res 2014;55:1192-214. [PubMed]
- Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays 2004;26:870-81. [PubMed]
- van Corven EJ, Groenink A, Jalink K, et al. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell 1989;59:45-54. [PubMed]
- Anliker B, Choi JW, Lin ME, et al. Lysophosphatidic acid (LPA) and its receptor, LPA1, influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia 2013;61:2009-22. [PubMed]
- Chen Y, Ramakrishnan DP, Ren B. Regulation of angiogenesis by phospholipid lysophosphatidic acid. Front Biosci (Landmark Ed) 2013;18:852-61. [PubMed]
- Lapierre DM, Tanabe N, Pereverzev A, et al. Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J Biol Chem 2010;285:25792-801. [PubMed]
- Liu YB, Kharode Y, Bodine PV, et al. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem 2010;109:794-800. [PubMed]
- Valet P, Pagès C, Jeanneton O, et al. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest 1998;101:1431-8. [PubMed]
- Ye X, Hama K, Contos JJ, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005;435:104-8. [PubMed]
- Goetzl EJ, Kong Y, Voice JK. Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol 2000;164:4996-9. [PubMed]
- Zhang Y, Chen YC, Krummel MF, et al. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J Immunol 2012;189:3914-24. [PubMed]
- Aoki J, Taira A, Takanezawa Y, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 2002;277:48737-44. [PubMed]
- Hosogaya S, Yatomi Y, Nakamura K, et al. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann Clin Biochem 2008;45:364-8. [PubMed]
- Stortelers C, Kerkhoven R, Moolenaar WH. Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics 2008;9:387. [PubMed]
- Chun J, Hla T, Lynch KR, et al. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 2010;62:579-87. [PubMed]
- Moolenaar WH, Hla T. SnapShot: Bioactive lysophospholipids. Cell 2012;148:378-378.e2.
- Choi JW, Herr DR, Noguchi K, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 2010;50:157-86. [PubMed]
- Yanagida K, Kurikawa Y, Shimizu T, et al. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta 2013;1831:33-41.
- Fotopoulou S, Oikonomou N, Grigorieva E, et al. ATX expression and LPA signalling are vital for the development of the nervous system. Dev Biol 2010;339:451-64. [PubMed]
- Koike S, Keino-Masu K, Ohto T, et al. Autotaxin/lysophospholipase D-mediated lysophosphatidic acid signaling is required to form distinctive large lysosomes in the visceral endoderm cells of the mouse yolk sac. J Biol Chem 2009;284:33561-70. [PubMed]
- Tanaka M, Okudaira S, Kishi Y, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 2006;281:25822-30. [PubMed]
- van Meeteren LA, Ruurs P, Stortelers C, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 2006;26:5015-22. [PubMed]
- Houben AJ, Moolenaar WH. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev 2011;30:557-65. [PubMed]
- Peyruchaud O, Leblanc R, David M. Pleiotropic activity of lysophosphatidic acid in bone metastasis. Biochim Biophys Acta 2013;1831:99-104.
- Gennero I, Laurencin-Dalicieux S, Conte-Auriol F, et al. Absence of the lysophosphatidic acid receptor LPA1 results in abnormal bone development and decreased bone mass. Bone 2011;49:395-403. [PubMed]
- Inoue M, Rashid MH, Fujita R, et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 2004;10:712-8. [PubMed]
- Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 2008;14:45-54. [PubMed]
- Dusaulcy R, Rancoule C, Grès S, et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res 2011;52:1247-55. [PubMed]
- Kremer AE, Martens JJ, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 2010;139:1008-18, 1018.e1.
- Yung YC, Mutoh T, Lin ME, et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 2011;3:99ra87 [PubMed]
- Nikitopoulou I, Oikonomou N, Karouzakis E, et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J Exp Med 2012;209:925-33. [PubMed]
- Wilkinson RN, van Eeden FJ. The zebrafish as a model of vascular development and disease. Prog Mol Biol Transl Sci 2014;124:93-122. [PubMed]
- Isogai S, Lawson ND, Torrealday S, et al. Angiogenic network formation in the developing vertebrate trunk. Development 2003;130:5281-90. [PubMed]
- Yaniv K, Isogai S, Castranova D, et al. Live imaging of lymphatic development in the zebrafish. Nat Med 2006;12:711-6. [PubMed]
- Jeltsch M, Tammela T, Alitalo K, et al. Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res 2003;314:69-84. [PubMed]
- Okuda KS, Astin JW, Misa JP, et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 2012;139:2381-91. [PubMed]
- Küchler AM, Gjini E, Peterson-Maduro J, et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol 2006;16:1244-8. [PubMed]
- Hogan BM, Bos FL, Bussmann J, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 2009;41:396-8. [PubMed]
- Shin K, Lee SH. Interplay between Inflammatory Responses and Lymphatic Vessels. Immune Netw 2014;14:182-6. [PubMed]
- Mulligan TS, Weinstein BM. Emerging from the PAC: studying zebrafish lymphatic development. Microvasc Res 2014;96:23-30. [PubMed]
- Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008;454:656-60. [PubMed]
- Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014;14:159-72. [PubMed]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999;98:769-78. [PubMed]
- Yang Y, García-Verdugo JM, Soriano-Navarro M, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 2012;120:2340-8. [PubMed]
- Hägerling R, Pollmann C, Andreas M, et al. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 2013;32:629-44. [PubMed]
- Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 2011;25:2187-97. [PubMed]
- Flores MV, Hall CJ, Crosier KE, et al. Visualization of embryonic lymphangiogenesis advances the use of the zebrafish model for research in cancer and lymphatic pathologies. Dev Dyn 2010;239:2128-35. [PubMed]
- Oliver G. Lymphatic vasculature development. Nat Rev Immunol 2004;4:35-45. [PubMed]
- Contos JJ, Ishii I, Fukushima N, et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol 2002;22:6921-9. [PubMed]
- Lai SL, Yao WL, Tsao KC, et al. Autotaxin/Lpar3 signaling regulates Kupffer's vesicle formation and left-right asymmetry in zebrafish. Development 2012;139:4439-48. [PubMed]
- Lee SJ, Chan TH, Chen TC, et al. LPA1 is essential for lymphatic vessel development in zebrafish. FASEB J 2008;22:3706-15. [PubMed]
- Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004;5:74-80. [PubMed]
- Lin CI, Chen CN, Huang MT, et al. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell Signal 2008;20:1804-14. [PubMed]
- Lin CI, Chen CN, Huang MT, et al. Lysophosphatidic acid up-regulates vascular endothelial growth factor-C and lymphatic marker expressions in human endothelial cells. Cell Mol Life Sci 2008;65:2740-51. [PubMed]
- Mu H, Calderone TL, Davies MA, et al. Lysophosphatidic acid induces lymphangiogenesis and IL-8 production in vitro in human lymphatic endothelial cells. Am J Pathol 2012;180:2170-81. [PubMed]
- Lin CE, Chen SU, Lin CC, et al. Lysophosphatidic acid enhances vascular endothelial growth factor-C expression in human prostate cancer PC-3 cells. PLoS One 2012;7:e41096 [PubMed]
- Sumida H, Noguchi K, Kihara Y, et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 2010;116:5060-70. [PubMed]
- Yukiura H, Hama K, Nakanaga K, et al. Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J Biol Chem 2011;286:43972-83. [PubMed]
- Chen JN, van Eeden FJ, Warren KS, et al. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development 1997;124:4373-82. [PubMed]
- Capdevila I, Izpisúa Belmonte JC. Knowing left from right: the molecular basis of laterality defects. Mol Med Today 2000;6:112-8. [PubMed]
- Raya A, Izpisúa Belmonte JC. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet 2006;7:283-93. [PubMed]
- Spéder P, Petzoldt A, Suzanne M, et al. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr Opin Genet Dev 2007;17:351-8. [PubMed]
- Essner JJ, Amack JD, Nyholm MK, et al. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 2005;132:1247-60. [PubMed]
- Hirokawa N, Tanaka Y, Okada Y, et al. Nodal flow and the generation of left-right asymmetry. Cell 2006;125:33-45. [PubMed]
- Raya A, Izpisúa Belmonte JC. Insights into the establishment of left-right asymmetries in vertebrates. Birth Defects Res C Embryo Today 2008;84:81-94. [PubMed]
- Yost HJ. Diverse initiation in a conserved left-right pathway? Curr Opin Genet Dev 1999;9:422-6. [PubMed]
- Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development 2003;130:2303-16. [PubMed]
- Schneider I, Houston DW, Rebagliati MR, et al. Calcium fluxes in dorsal forerunner cells antagonize beta-catenin and alter left-right patterning. Development 2008;135:75-84. [PubMed]
- Hecht JH, Weiner JA, Post SR, et al. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol 1996;135:1071-83. [PubMed]
- Yung YC, Stoddard NC, Mirendil H, et al. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015;85:669-82. [PubMed]
- Contos JJ, Fukushima N, Weiner JA, et al. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A 2000;97:13384-9. [PubMed]
- Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007;316:295-8. [PubMed]
- Kihara A, Igarashi Y. Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim Biophys Acta 2008;1781:496-502.
- Mitra P, Oskeritzian CA, Payne SG, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A 2006;103:16394-9. [PubMed]
- Sato K, Malchinkhuu E, Horiuchi Y, et al. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem 2007;103:2610-9. [PubMed]
- Kawahara A, Nishi T, Hisano Y, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009;323:524-7. [PubMed]
- Osborne N, Brand-Arzamendi K, Ober EA, et al. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr Biol 2008;18:1882-8. [PubMed]
- Murata N, Sato K, Kon J, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J 2000;352:809-15. [PubMed]
- Ishii I, Fukushima N, Ye X, et al. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 2004;73:321-54. [PubMed]
- Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat 2004;73:141-50. [PubMed]
- Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J 2002;16:1874-8. [PubMed]
- Jaillard C, Harrison S, Stankoff B, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci 2005;25:1459-69. [PubMed]
- Burchill MA, Yang J, Vang KB, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 2008;28:112-21. [PubMed]
- Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355-60. [PubMed]
- Pham TH, Baluk P, Xu Y, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 2010;207:17-27. [PubMed]
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 2007;115:84-105. [PubMed]
- Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets 2007;8:155-67. [PubMed]
- Foster CA, Mechtcheriakova D, Storch MK, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol 2009;19:254-66. [PubMed]
- Haghikia A, Gold R. Sphingosine-1-phosphate and its receptors as a possible therapeutic target in autoimmune diseases of the nervous system. J Neuroimmunol 2010;218:1-2. [PubMed]
- Marsolais D, Rosen H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat Rev Drug Discov 2009;8:297-307. [PubMed]
- Miron VE, Ludwin SK, Darlington PJ, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 2010;176:2682-94. [PubMed]
- Miron VE, Schubart A, Antel JP. Central nervous system-directed effects of FTY720 (fingolimod). J Neurol Sci 2008;274:13-7. [PubMed]
- Nayak D, Huo Y, Kwang WX, et al. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience 2010;166:132-44. [PubMed]
- Pébay A, Toutant M, Prémont J, et al. Sphingosine-1-phosphate induces proliferation of astrocytes: regulation by intracellular signalling cascades. Eur J Neurosci 2001;13:2067-76. [PubMed]
- Rouach N, Pébay A, Même W, et al. S1P inhibits gap junctions in astrocytes: involvement of G and Rho GTPase/ROCK. Eur J Neurosci 2006;23:1453-64. [PubMed]
- Yamagata K, Tagami M, Torii Y, et al. Sphingosine 1-phosphate induces the production of glial cell line-derived neurotrophic factor and cellular proliferation in astrocytes. Glia 2003;41:199-206. [PubMed]
- Forrest M, Sun SY, Hajdu R, et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther 2004;309:758-68. [PubMed]
- Koyrakh L, Roman MI, Brinkmann V, et al. The heart rate decrease caused by acute FTY720 administration is mediated by the G protein-gated potassium channel I. Am J Transplant 2005;5:529-36. [PubMed]
- Mazurais D, Robert P, Gout B, et al. Cell type-specific localization of human cardiac S1P receptors. J Histochem Cytochem 2002;50:661-70. [PubMed]
- Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 2010;9:883-97. [PubMed]
- Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta 2002;1582:222-7. [PubMed]
- Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 2003;102:3665-7. [PubMed]
- Mizugishi K, Yamashita T, Olivera A, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 2005;25:11113-21. [PubMed]
- Skoura A, Michaud J, Im DS, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol 2011;31:81-5. [PubMed]
- Mendelson K, Evans T, Hla T. Sphingosine 1-phosphate signalling. Development 2014;141:5-9. [PubMed]
- Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 2000;106:951-61. [PubMed]
- Kono M, Mi Y, Liu Y, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 2004;279:29367-73. [PubMed]
- Mendelson K, Lan Y, Hla T, et al. Maternal or zygotic sphingosine kinase is required to regulate zebrafish cardiogenesis. Dev Dyn 2015;244:948-54. [PubMed]
- Hisano Y, Inoue A, Okudaira M, et al. Maternal and Zygotic Sphingosine Kinase 2 Are Indispensable for Cardiac Development in Zebrafish. J Biol Chem 2015;290:14841-51. [PubMed]
- Kupperman E, An S, Osborne N, et al. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 2000;406:192-5. [PubMed]
- Hisano Y, Inoue A, Taimatsu K, et al. Comprehensive analysis of sphingosine-1-phosphate receptor mutants during zebrafish embryogenesis. Genes Cells 2015;20:647-58. [PubMed]
- Stainier DY, Fouquet B, Chen JN, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996;123:285-92. [PubMed]
- Rossi A, Kontarakis Z, Gerri C, et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015;524:230-3. [PubMed]
- Hwang WY, Fu Y, Reyon D, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 2013;31:227-9. [PubMed]


