
Improving the precision and performance of proton pencil beam scanning
Introduction
Due to the finite range of particle beams and the excellent dose localization in depth with a characteristic dose maximum at the location where the beam stops, the so-called Bragg peak, proton and ion therapy provide an improved capability of shaping the dose conformally to the target volume with a significant dose sparing of healthy tissues surrounding the tumour as compared to conventional therapy with photons.
Another advantage of charged particle beams is given by the possibility to scan a small pencil beam laterally by magnetic deflection and in depth by changing the energy of the beam as a method for painting the dose dynamically. The Paul Scherrer Institut (PSI) has committed itself to the development of pencil beam scanning for over three decades, starting in the 80s with pion beams (1), in 1992 with the realization of the first proton scanning gantry in the world (Gantry 1) operational since 1996 (2) and recently with the installation of a new prototype (3) Gantry 2, which is currently being commissioned (4). The goal is to bring the scanning technology close to the physical limits using a new next generation gantry. By providing new advanced beam delivery techniques we should expand the spectrum of the clinical applications treated with pencil beam scanning to include moving targets. This shall be the main goal of our centre in terms of translational research from accelerator physics to clinics over the next decade.
The main topic of this report is to present the technical characteristics of the new Gantry 2 in view of the potential of this system for new future translational research.
In Section 2, we briefly describe the history and the technical status of the proton facility at PSI with emphasis on the technical features of the first prototype Gantry 1, which provided the necessary experience and background for the current and future developments of Gantry 2.
In section 3, we discuss why further developments of proton pencil beam scanning are needed.
Section 4 is focused on the conceptual design of the new Gantry 2.
Section 5 presents the advancement of the scanning technology for achieving very fast scanning techniques designed for treating moving targets with scanning in conjunction with image guidance.
In section 6, we briefly mention the potential clinical indications for Gantry 2.
Conclusions can be found in section 7.
Scanning experience with the first prototype Gantry 1
The technological innovation of PSI in the field of particle therapy in the 90s was the introduction of pencil beam scanning, a method where narrow pencil beams are superimposed laterally and in depth to achieve a dose distribution conformal to the target volume while sparing the surrounding healthy tissue as much as possible (2).
The first prototype implementing this technique is named PSI’s Gantry 1. During the past 16 years, more than 800 patients have been treated successfully, most of them for tumours in the skull base region. PSI developed at Gantry 1 and introduced in the clinical program intensity modulated proton therapy (IMPT) (5), the equivalent of intensity modulated therapy with photons (IMRT). A paediatric program was started in 2000 in collaboration with the University of Zurich, based on the idea that children should profit most from proton therapy. Children under the age of 5 years are treated under anaesthesia. Being a great success, this program now makes up one third of all patient treatments.
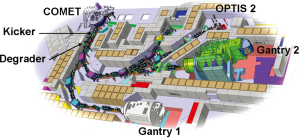
Gantry 1 was designed for parasitic operation at PSI’s 590 MeV ring cyclotron. By connecting the Gantry 1 beam line to a new dedicated cyclotron COMET (6) in 2006 (Figure 1), two major limitations of the first decade of clinical operation were eliminated, namely considerable current instabilities of the split and degraded beam and long yearly shutdowns due to the maintenance of the main accelerator of PSI. Since its introduction, COMET has been operating reliably with patients treated at 5 days a week all year long and without shutdowns longer than 3 days.
Gantry 1 implements discrete spot scanning where the beam is turned off while moving from one spot position to the next. This approach allows controlling the spot parameters like position and dose under static conditions and was chosen to cope with the beam instabilities present during the first years of operation. Individual spots of variable length are applied at a rate of up to 200 spots/s.
The fine adjustment of the proton energy is achieved with a so-called range shifter, a device located in the gantry nozzle in front of the patient, consisting of 40, 4.5 mm water equivalent, polyethylene plates which can be inserted individually in the beam. This method has the advantage of working with an invariant fixed depth dose profile, but multiple Coulomb scattering (MCS) of the protons in the range shifter plates make the beam width very sensitive to the air gap between nozzle and the patient surface and can lead to a fast degradation of the lateral dose fall-off with increasing nozzle-patient distance (7).
Laterally, the beam is scanned magnetically along one axis with a sweeper magnet located upstream from the last 90° bending magnet. With such an upstream scanning mode, the beam optics can be designed to provide parallel scanned pencil beams at the target location (apparent source at the infinite). The second lateral scan axis is realized by moving the patient, i.e., the target volume, with the patient-table. The speed of this mechanical movement is limited and contributes substantially to the overall dead-time of the system. The scanning system is Cartesian in all three dimensions.
The scan sequence to move the beam on a 3D-grid of 5 mm step size is dictated by the scan speed of the three axis: lateral with sweeper magnet (5 ms/step), in-depth with range shifter (50 ms/step) and lateral with patient-table (1 s/step). The resulting time to treat a 1 litre volume with 2 Gy with discrete spot scanning is 3 min with a duty factor of 50% (Table 1). Due to the use of the motion of the patient-table as a scanning axis, substantial volumetric repainting (defined later) with this scanning performance is not practical and therefore Gantry 1 is limited in its application to non-moving targets.
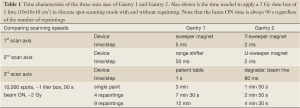
Full table
Motivation for further developments in spot scanning
To understand the reasons to further push the pencil beam scanning technology we must first compare this technology with the more traditional method of passive scattering, which is based on a uniform scattered broad beam and where the dose is shaped with fixed collimators and compensators.
The major advantages of scanning, as we have learned from Gantry 1 (8), are the following:
With scanning one can provide a true 3D-dose conformation with variable modulation of the range as opposed to fixed range modulation of scattering, which delivers unnecessary full dose outside the target. With scanning there is no need to fabricate and mount patient-field specific hardware in the beam line in front of the patient. One can thus deliver multiple fields in sequence without the need for personnel to enter the room to change equipment in between fields. This reduces treatment time and makes the use of many field directions easier.
Scanning can deliver not only homogeneous dose fields [so called single field uniform dose, i.e., SFUD (9)] but can also provide non-homogeneous dose distributions with planned dose shaping within the target (non-uniform dose fields NUDF). Planned non-homogenous dose fields can be combined within a simultaneous optimization of fields to obtain superior dose distributions (multiple field optimization). In the 90s this approach was named intensity modulated proton therapy to indicate its similarity with IMRT. IMPT has been pioneered at our institute with the Gantry 1 system, where it has been the only IMPT capable system for over a decade (5).
With scanning, all protons in the pencil beam are stopped in the tumour, hence, scanning provides the best possible efficiency of utilization of the beam. This results in a lower neutron background (10) and a lower activation of elements in the nozzle and in the whole beam line.
Other points to be more explicitly demonstrated with the new Gantry 2 system are the following:
Planned NUDF, delivered by pencil beam scanning, should be used in the future for providing biological dose targeting (dose shaped within the target according the biologically measured distribution of tumour activity).
Scanning is usually delivered without placing individual hardware in the beam line in front of the patient, while scatterers and compensators are needed with scattering. Since the amount of material in the nozzle is almost negligible — Gantry 2 works with variable beam energy instead of a range shifter — in general we expect to have a sharper lateral dose fall-off as with scattering. At low energies (<100 MeV), the use of collimation added on top of conformal scanning is possibly the best solution. Both alternatives, scanning alone for deep seated tumours and scanning in combinations with collimation for shallower depths, are expected to be superior to scattering.
With scanning it should be easier to provide more robust field patching techniques (against inter-field shifts) than with scattering by adding adjacent fields with overlapping smooth dose transition regions.
Presently the major disadvantage of scanning is its specific sensitivity to organ motion; a problem common to any dynamic therapy including IMRT in conventional therapy. This issue was already anticipated in the early 90 s with Gantry 1 (11). Interferences of the motion of the target with the motion of the beam can produce significant dose errors spoiling the homogeneity of the dose distribution within the target. At PSI this is the main reason why we have only been able to treat non-moving target to date (tumours in the head, spinal chord and lower pelvis).
A possible remedy to the organ motion problem of scanning is to realize much faster scanning techniques in order to apply the dose to the target very quickly and as repeatedly as possible (repainting), approaching as much as possible the repainting capability of scattering, which is the basis of the success of this technique in the context of organ motion.
Other methods under consideration aim at reducing the extent of organ motion itself by synchronizing dose delivery with a given phase interval of the breathing cycle (gating). This approach not only reduces the dose homogeneity errors within the target, but also allows reducing the safety margins at the border of the tumour, at the potential cost of increasing treatment delivery time.
The motion-reduction methods being discussed at PSI are based on scanning in connection with breath hold - gating - or tracking techniques. A very fast scanning technique should in any case help for repainting the target repeatedly within the same daily session with any of these approaches (12).
Although scanning will never reach the same rate of repainting as scattering, it could represent a better approach to image-guided proton therapy, since it can provide a tighter conformation of the dose to the target with a beam delivery method, which can be adapted very quickly to the instant target motion. During beam delivery tumour-motion tracking could be done by shifting the position of the following in real time: spots, whole irradiated energy layers and/or whole irradiated volume.
The new scanning gantry prototype at PSI: Gantry 2
To overcome the limitations of Gantry 1, Gantry 2 was designed with the goal of much faster scanning to support volumetric dose repainting and a smaller spot size to improve the lateral dose fall-off.
Technical specification
A substantial improvement of the beam spot size is achieved by avoiding the use of a range shifter in the nozzle. In Gantry 2 the proton range is controlled by adjusting the beam energy dynamically with the degrader system and the beam line upstream from the gantry. This approach minimizes the material and the associated MCS in the nozzle, but also results in range dependent depth dose profiles. Special care has been taken to make the beam energy changes as fast as possible by using low mass multiple carbon wedges in the degrader and laminated magnets for the beam line. This allows beam energy changes in 80 ms for typical range steps of 5 mm water equivalent.
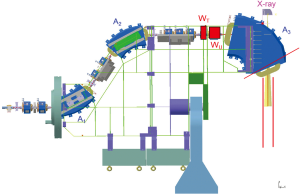
The scanning speed is dramatically improved over that of Gantry 1 by scanning both lateral axes magnetically. As in Gantry 1, the sweeper magnets are installed upstream from the last bending magnet, which is designed such that the scanned pencil beams are parallel in both directions at the iso-centre over a scanning area of 12×20 cm2. This upstream parallel scanning (Figure 2) not only allows a compact gantry design but also reduces the complexity of therapy planning. This is especially the case for large effective field sizes where multiple scanning areas can be patched together with overlapping regions with smooth dose gradients to render the patching insensitive to target motion.
The sweeper magnets scan the beam at the iso-centre with a speed of 2 cm/s along one axis (transverse to the magnets, referred to as T-axis) and 0.5 cm/s along the other axis (dispersive direction, referred to as U-axis). The overall dead-time between spots given by setting the sweepers, verifying spot parameters and logging data is 2 ms per 5 mm step. Due to the higher speed of the lateral motion compared to the changes of the range, the dose is applied in iso-energy layers. The faster scanning of Gantry 2 results in a dead-time of 20 s for the 2 Gy 1 litre box single painted spot scanning example compared to the 90 s of Gantry 1 (Table 1), which makes moderate volumetric repainting feasible already in the simplest discrete spot scanning mode.
Gantry 2 will start its clinical operation with the discrete spot scanning mode analogous to Gantry 1. However, the goal of Gantry 2 is to demonstrate the potential of new scanning methods by pushing the scanning speed to the physical limits with new delivery techniques like line or contour scanning. From start the control system was designed for highest flexibility in order to support both clinical operation and new research projects, including future developments. It is based on two independent systems: a delivery system which steers the dose application (beam energy, lateral beam position, beam current and dose) and a verification system to measure and verify these critical parameters by independent detectors and diverse methods.
System realization
The mechanical and room layout of Gantry 2
During the conceptual phase particular attention was given to the gantry- and room-layout to allow an effective patient-handling and the installation of modern imaging equipment.
The mechanical layout of Gantry 2 is characterized by a gantry rotation ranging from –30° to 180°. Since the missing degrees can be easily compensated by rotating the patient-table this solution does not compromise the choices of the incident beam angles. The important advantage of this configuration is the integration of a fixed false floor covering the gantry pit (with exception of the rolling cover of the slit where the nozzle rotates) which permits easy access to the patient in every treatment situation (Figure 3). The medical-staff can have a direct and close contact with the patient for reassurance or rescue in case of emergency. This layout also simplifies the work of the medical physicists and developers, who can easily install their equipment on top of the patient-table and effectively verify that the equipment is correctly positioned.

We are pleased to observe that this layout has been recently adopted by several vendors in the proton therapy field.
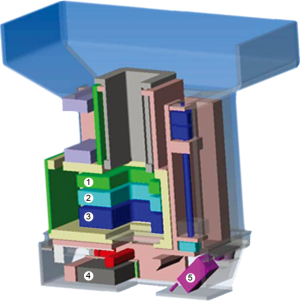
The nozzle (Figure 4) was specifically designed to improve the precision of the treatment by keeping the pencil beam size as small as possible. The short distance between vacuum window and iso-centre of 86 cm reduces the effect of scattering in air without compromising beam size at low energies. The three monitors in the nozzle (two dose-monitors and one strip chamber for measuring beam position) are fixed to a movable support that can be extracted to reduce the air-gap between nozzle and patient with a range of motion of 27 cm. On the same support, a 2.5 cm thick graphite pre-absorber is mounted just before the nozzle exit-window and can be moved in and out of the beam as desired under remote control. Beam size, and in turn lateral penumbra, can therefore be minimized by extracting the nozzle so to reduce the effect of scattering in the monitors and particularly in the pre-absorber. If required, collimators/apertures can be mounted on the nozzle to improve furthermore the lateral penumbra for shallow tumours. The slim shape of the nozzle additionally helps to reduce the air-gap. All included we have shown that the beam size (sigma) in air can be kept below 5 mm for all energies down to 70 MeV (Figure 5).
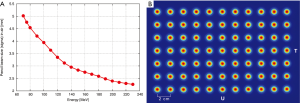
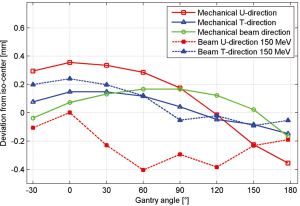
The mechanical support of the gantry has been designed to be very robust and reliable in order to offer reproducible movements. Therefore, one very important check is to measure the mechanical deformation of the gantry for different gantry angles. Measurements of the deviation from the ideal iso-centre are shown in Figure 6 and are within a window of 0.8 mm for all angles. This mechanical behaviour is reflected in the achieved accuracy of the measured beam position for different gantry angles. If required the residual position-errors can be further improved with angle-dependent beam tuning corrections. Three fan lasers (LAP lasers) mounted on the walls of the treatment room are aligned to the mechanical iso-centre and to the three room-axes. The mechanics for the extraction of the nozzle has also shown to be very accurate so that additional lasers could be mounted in the nozzle itself and aligned to the room-lasers at iso-centre. Both room- and nozzle-lasers are the main instrument used to position the dosimetric equipment.
The imaging equipment in the treatment room
The large floor surface of over 50 m2 provides a comfortable working environment making it possible to install an in-room CT for patient setup and verification as shown in Figure 3.
The installed Siemens Sensation Open CT-on-Rail has a large bore (82 cm) and a 24/40-slice configuration with 4DCT capability. The particularity of the equipment is that the CT gantry moves on rails while the table stays still during image acquisition. Therefore the same patient table used for proton irradiation can be used for CT acquisition. After positioning, a simple table rotation brings the patient from the CT to the treatment position. If space is not a concern we are convinced that CTs are the best solution for an in-room patient positioning rather than C-arms, cone-beam CTs or orthogonal X-rays. As a matter of fact, besides offering 3D volumetric matching of soft-tissue and bony structures, CT images allow us to perform accurate dose calculations to verify dosimetrically the impact of patient positioning corrections. This is an important advantage compared to cone-beam CTs.
The other innovative imaging approach with Gantry 2 is the installation of a Beam’s-Eye-View (BEV) X-ray fluoroscopy system, which will be used to verify patient positioning and is part of a grant study for treating moving-targets. The X-ray tube is mounted on the back of the last bending magnet and X-rays are shined through a hole in the return yoke along the proton direction (Figure 2). This realisation was possible only because the last bending magnet has a large gap due to the choice of upstream scanning. The 150 kV X-ray tube can be operated in fluoroscopy mode and the field size at the iso-centre is 20×25 cm2. The digital flat panel (Varian PaxScan 4020E) is mounted on an extractable support (Figure 3). Similar to a portal imaging device on a conventional linac, the BEV can acquire X-ray images synchronised with proton irradiation and provide information of the transversal location of the tumour and the nearby bony structures. This information can be used for image-guided radiation therapy to gate, track, or QA the beam delivery.
Patient-handling and remote control
The control room for both Gantry 2 and the imaging equipment is located outside the treatment room. The aim is to operate all moving components, such as patient-table and gantry-rotation, remotely as well as patient positioning and beam delivery, in order to improve efficiency. To prevent harmful movement under safety regulation the gantry patient positioning system defines virtual walls recognized by the system itself and uses collision plates around the nozzle. In addition, several dome cameras are installed in the room, which can observe the patient under different angles.
Development of advanced scanning technique: preliminary results
Compared to the traditional scattering technique the major disadvantage of the scanning technique is the increased sensitivity to organ motion. Moving the target during the application of individual spots can disturb the homogeneity of the dose distribution. Several mitigation techniques are proposed and discussed e.g., gating, repainting, tracking and breath-hold (13,14).
For us, one of the most promising approaches to tackle organ motion is repainting in combination with - when needed - gating or breath-hold (12). The basic idea of repainting is that the full dose distribution of an irradiation is applied repeatedly in several iterations such that possible interferences of neighbouring spots are statistically smeared out. The dose per spot is reduced according to the number of repaintings in order to get the same final dose distribution.
Thus, one of the preconditions for efficient repainting is to have very fast scanning with minimal dead-time. Most of the dead-time of discrete spot scanning can be avoided by painting the dose continuously along lines, meanders or contours. The most efficient mode should be achieved by painting the dose of a whole energy-layer without interrupting the beam delivery. With the help of the sweeper magnets and a typical line separation of 0.5 cm, a rectangular energy layer of 10×10 cm2 can be painted in continuous mode as fast as 125 ms at maximum speed. For precise dose painting one can shape the dose by changing the velocity of moving the beam position and/or by changing dynamically the intensity of the beam. These are the major topics which we plan to explore in the future developments of Gantry 2.
In order to provide a full conformal dose distribution it is also necessary to provide a varying proton flux along the scanned lines. For a typical target the proton flux along one line can vary up to a factor of 30.
A vertical deflector plate and several collimators were installed inside the proton accelerator close to the ion source so as to provide a very fast modulation of the beam intensity during scanning. Near the source the protons still have a very low kinetic energy and can thus be quickly deflected with an electrostatic field. Only the fraction of the initial beam emerging from the collimation system is then accelerated. By changing the voltage of the deflector plate it is thus possible to modulate the proton beam current in a reproducible and very fast way on the time scale of 100 µs.
A particular challenge in continuous scanning is the precise control of the dose along painted lines. Since the vertical deflector plate has the shortest latency in the whole system we decided to install a feed-back control loop acting on the vertical deflector and based on the signal of the primary dose monitor right in front of the patient. In this so-called time driven configuration a line scan runs completely deterministic according to a given table containing time, position and intensity data.
It should be remembered that 3D-conformal scanning requires the delivery of non-homogeneous proton fluences within an energy layer.
With Gantry 2 we have two complementary methods of painting non-homogeneous dose lines. Either the scan speed of the sweeper is kept constant and the intensity of the proton beam current follows the shape of the dose profile (intensity modulation), or the speed of the sweeper is modulated and the proton current is kept constant (speed modulation). A combination of those is possible as well.
Speed modulation at maximum beam current is most efficient for reducing treatment time since it works with a fully extracted beam. However critical situations could arise in this mode when portions of the dose line profile must be delivered, which are below the limit given by the maximum velocity of the sweepers.
Intensity modulation at maximum sweeper speed can easily handle very low doses but in turn it is limited by the maximum dose which can be delivered at maximum speed. The limit is given here by the maximal available extracted beam current.
In practice a combination of both modes will be used to get the most flexible and effective scan algorithm. Speed modulation is open-ended for modulating the dose on the high-dose side while intensity modulation better covers the low-dose side.
We intend to develop several repainting strategies. One of the most important characterizations is regarding the detailed sequence of changing the beam energy. For the so-called layer repainting mode, iso-energy layers are repeated in sequence without changing the beam energy which is set only once. For the so-called volumetric repainting each energy layer is single painted and the whole target volume is repainted several times including intermediate energy changes.
From simulations we feel confident that volumetric repainting brings additional benefits compared to layer repainting (12). However, volumetric repainting can be effectively implemented only if the beam energy can be switched rapidly. Fast energy change has been one of the main requirements for our facility. Due to dedicated power supplies for the magnets, laminated magnets and a fast mechanical degrader system we can provide energy switching times of about 80 ms for typical energy steps of 3 MeV.
Thanks to these fast energy changes we are also able to implement a very fast uniform scanning (iso-energy layers with uniform flux). The 3D- dose shaping can be achieved by using individual collimator-compensator pairs mimicking the method used with passive scattering. In an experiment (15), we were able to conformally irradiate a 1 litre target with 1 Gy with 48 repaintings applied on the most distal layer in 30 s and thereby getting closer to the repainting conditions of the scattering technique. The flexibility of the scanning system brings additional advantages, e.g., better proximal conformity to the target volume. We could show that scanning can simulate scattering fairly well including variable modulation of the range. The opposite is not true, since scattering cannot simulate scanning and cannot provide IMPT.
The main focus of the new developments with Gantry 2 remains however the delivery of fast conformal line scanning with volumetric repainting to cope with moving organs. In another experiment, a spherical target of 0.5 litre was irradiated with 0.1 Gy in pure line scanning mode. 18 different proton energies were delivered in sequence. The overall scan was completed within 6 s (Figure 7). To get the typical field dose of 1 Gy the whole scan sequence must be repeated 10 times. This is equivalent to 10 volumetric repaintings and would require only 60 s.
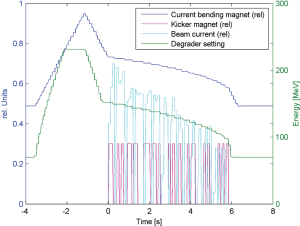
The possibility to irradiate the whole target volume within 5-10 s opens the door to the idea of treating small lung nodules as a whole with scanning within a single breath hold, and to repeat the treatment many times on the same daily fraction. This could include image guidance applied to the whole volume. The idea would be to take advantage of the increased scanning speed of Gantry 2 for reducing a complex dynamic treatment into a simple sequence of fully applied static treatments.
Possible clinical indications to be treated with Gantry 2
Static tumours will be treated on Gantry 2 with discrete spot scanning as with Gantry 1, but with improved dose precision by using smaller pencil beam sizes. The main improvement results from the dynamic variable energy of the beam line of Gantry 2. Brain tumours, head and neck targets and tumours near the spinal cord could particularly benefit from this improvement.
For very superficial tumours we will provide the option to use scanning with added collimation. One could think to use this approach also for eye treatments, e.g., for retinoblastoma and for treating children under anaesthesia in supine position. The use of a gantry in horizontal position with a patient chair coupled to the patient table could then be seen as an alternative of building an extra horizontal beam line dedicated to eye treatments at new proton therapy facilities.
We also expect to be able to treat moving targets with scanning. Moving targets in the trunk will be treated by applying multiple repaintings (tumours in the lower pelvis like rectum, cervix, pancreas - or breast tumours with lymph nodes involvement). Lung and liver could be treated by repeating whole dose volumes painted within a single breath-hold (but gating and tracking could be developed and used as well if necessary).
Very large tumours like medulloblastoma are planned to be treated in one sequence by making use of the remote-controlled patient-table and by taking advantage of the two dimensional parallelism of the scanned beam.
In the end the overall goal is to provide a system which is potentially capable of treating any valid indication for proton therapy with a well-designed basic scanning system with minimal hardware.
Conclusions
Over the years PSI has contributed very substantially to the development of the field of proton therapy by introducing the first conformal proton pencil beam scanning system in the world. In this context we would like to mention the work of our Japanese colleagues in the 80 s with low energy scanning proton beams (16) and the parallel work of GSI with scanning ion beams (17). The first scanning system of PSI has been realized on a very compact gantry (Gantry 1), capable of delivering multiple fields in one go. The experience with this system has been very positive, especially in the context of delivering simultaneous field optimization, i.e., IMPT. Today IMPT is considered a necessary development for being able to compete with IMRT in conventional therapy. As a result of these developments, the whole community and the industrial providers of proton therapy systems are now switching to scanning beams.
There is, however, still a lot of new scanning developments which are potentially worth being done. To this goal we have developed Gantry 2 capable of delivering scanning with a much higher speed and with enhanced capability to adapt the dose delivery to image guidance and to cope also with the motion of internal organs. In this report, we have presented the technical features of the new system and sketched the main ideas for potential clinical applications of scanning. The goal has been to design a system capable of delivering the dose with the highest possible precision for treating essentially any clinical indication including moving targets and for providing biological dose targeting.
The Gantry 2 system has been realized with minimal dose shaping and monitoring equipment in order to have a flexible and fully software-based approach to proton beam delivery. We believe that this technology will render passive scattering obsolete, also in view of the fact that scattering can be replaced with fast highly repainted uniform scanning techniques.
The scope of the Gantry 2 project is to bring, very similarly as with Gantry 1 in the past, a next significant contribution into the future of the field of proton therapy.
Acknowledgments
We would like to thank the entire team of the Center of Proton Therapy of PSI for their engagement.
Some of the ideas presented in this report are protected by patent applications. As a government institution, our institute is not involved in providing commercial systems.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Huan Giap and Eric Y Chuang) for the series “Particle Beam Therapy I” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.10.08). The series “Particle Beam Therapy I” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pedroni E. Therapy planning System for the SIN Pion Therapy Facility. In: Burger G, Broerse JJ. eds. Treatment planning for external beam therapy with Neutrons. Munich: Urban & Schwarzenberg, 1981:60-9.
- Pedroni E, Bacher R, Blattmann H, et al. The 200-MeV proton therapy project at the Paul Scherrer Institute: conceptual design and practical realization. Med Phys 1995;22:37-53. [PubMed]
- Pedroni E, Bearpark R, Böhringer T, et al. The PSI Gantry 2: a second generation proton scanning gantry. Z Med Phys 2004;14:25-34. [PubMed]
- Pedroni E, Meer D, Bula C, et al. Pencil beam characteristics of the next-generation proton scanning gantry of PSI: design issues and initial commissioning Results. Eur Phys J Plus 2011;126:66.
- Lomax AJ, Boehringer T, Coray A, et al. Intensity modulated proton therapy: a clinical example. Med Phys 2001;28:317-24. [PubMed]
- Schippers JM, Dölling R, Duppich J, et al. The SC cyclotron and beam lines of PSI’s new protontherapy facility PROSCAN. Nucl Instr Meth B 2007;261:773-6.
- Lomax AJ, Böhringer T, Bolsi A, et al. Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med Phys 2004;31:3150-7. [PubMed]
- Pedroni E, Scheib S, Böhringer T, et al. Experimental characterization and physical modelling of the dose distribution of scanned proton pencil beams. Phys Med Biol 2005;50:541-61. [PubMed]
- Lomax AJ. Intensity Modulated Proton Therapy. In: Delaney T, H Kooy H. eds. Proton and charged particle radiotherapy. Boston: Lippincott, Williams and Wilkins, 2008.
- Schneider U, Agosteo S, Pedroni E, et al. Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys 2002;53:244-51. [PubMed]
- Phillips MH, Pedroni E, Blattmann H, et al. Effects of respiratory motion on dose uniformity with a charged particle scanning method. Phys Med Biol 1992;37:223-34. [PubMed]
- Zenklusen SM, Pedroni E, Meer D. A study on repainting strategies for treating moderately moving targets with proton pencil beam scanning at the new Gantry 2 at PSI. Phys Med Biol 2010;55:5103-21. [PubMed]
- Knopf A, Bert C, Heath E, et al. Special report: workshop on 4D-treatment planning in actively scanned particle therapy--recommendations, technical challenges, and future research directions. Med Phys 2010;37:4608-14. [PubMed]
- Rietzel E, Bert C. Respiratory motion management in particle therapy. Med Phys 2010;37:449-60. [PubMed]
- Zenklusen SM, Pedroni E, Meer D, et al. Preliminary investigations for the option to use fast uniform scanning with compensators on a gantry designed for IMPT. Med Phys 2011;38:5208-16. [PubMed]
- Kawachi K, Kanai T, Matsuzawa H, et al. Three dimensional spot beam scanning method for proton conformation radiation therapy. Acta Radiol Suppl 1983;364:81-8. [PubMed]
- Haberer T, Becher W, Schardt D, et al. Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods Phys Res 1993;330:296-305.


