
Pathology of pancreatic tumors
Pancreatic ductal adenocarcinoma (PDA)
Introduction
Infiltrating ductal adenocarcinoma is the most common type of carcinoma of the pancreas. The incidence of ductal adenocarcinoma is higher in Western industrialized countries than in undeveloped countries. However, in terms of ethnic groups, it is more common in the Maoris in New Zealand, in native Hawaiians, and in African-Americans living in the United States. Not only is the incidence in African Americans higher than in whites in the United States, but their cancers tend to present at a higher stage and are less often surgically resectable than are cancers in Caucasians. Age is a significant risk factor for pancreatic cancer. The peak incidence of the disease is in the 7th to 8th decades of life, and most cases (80%) occur between ages 60 and 80 years. For young patients under the age of 20 with pancreatic adenocarcinoma, they commonly have other associated genetic known risk factor, such as Peutz-Jeghers syndrome (PJS). Pancreatic cancer is more common in men than in women.
Several environmental risk factors have been implicated in the risk of pancreatic cancer, including tobacco use, diet, alcohol, and high caloric intake. Cigarette smoking has been consistently identified as risk factor for pancreatic cancer (1). A number of dietary factors are associated with pancreatic cancer. Diets high in meat, pork products, fats, nitrates increases the risk. The regular use of aspirin has been associated with a reduced risk of pancreatic cancer in some studies. Obesity and physical inactivity increase the risks. Case-control studies have suggested an association between certain occupations and the development of pancreatic cancer. These include workers exposed to coal gas, metal workers, workers in the leather tanning industry, and dry cleaners. Exposure to certain chemicals, including beta-naphthylamine, benzidine, solvents, dichlorodiphenyltrichloroethane (DDT), and gasoline may also increase the risk. Several medical conditions also increase the risk of pancreatic cancer. These include long standing diabetes mellitus and chronic pancreatitis. Several studies have found an increased risk in patients who underwent cholecystectomy or partial gastrectomy.
Operative resection is the only potentially curative treatment for pancreatic cancer, yet only 15 to 20 percent of patients are candidates for pancreatectomy at the time of diagnosis. Routine screening is not usually done in average-risk individuals given the low incidence of pancreatic cancer and the lack of low cost affordable test with high sensitivity and specificity. However, those with an elevated risk of pancreatic cancer, such as those with hereditary pancreatitis and some inherited cancer susceptibility syndromes, would benefit more from screening to detect early pancreatic lesions. There are two recognized precursor lesions for the development of pancreatic adenocarcinoma: intraductal papillary mucinous neoplasm (IPMN) and high-grade pancreatic intraepithelial neoplasia (PanIN). In this review, we would like to discuss the pathologic features including the gross findings, histopathologic findings, and molecular alterations for pancreatic adenocarcinoma, mucinous neoplasm, and other carcinoma of the pancreas.
Pathologic features
Grossly
The majority of ductal adenocarcinoma arises in the head of the pancreas, less frequently it arises in the body or tail. Ductal adenocarcinoma can range in size from microscopic disease to masses over 10 cm. On cut section, most are firm, stellate, poorly defined, and white-yellow. In some cases the carcinoma may be difficult to distinguish grossly from areas of fibrosis in chronic pancreatitis (2). Most PDA are solid, but some can form cysts. Ductal carcinoma of the head of pancreas can cause stenosis of the distal common bile duct and the bile duct proximal to this area is dilated. Most ductal adenocarcinoma invade into retroperitoneal structures, and large tumor can invade the duodenum, peritoneum, stomach, transverse colon, jejunum and even gallbladder. Tumors arising in the tail can infiltrate into the spleen, adrenal glands and intestines.
Histopathology
Ductal adenocarcinoma is invasive, gland-forming, epithelial neoplasms that commonly elicit a marked desmoplastic reaction. The degree of differentiation ranges from well-formed glands to poorly oriented cells infiltrating singly or forming solid sheets. The infiltrating glands are distributed in a disorganized fashion. It is typical to see well differentiated glands and low grade architecture and then have significant cytologic atypia (Figure 1). The infiltrating glands of ductal adenocarcinoma grow in a haphazard fashion, violating the usual branching of benign glands. The normal pancreatic architecture is acini surrounding the ducts and separated by vessels. The glands of infiltrating adenocarcinoma are disorganized and can be found adjacent to vessels, without intervening acini or stroma.
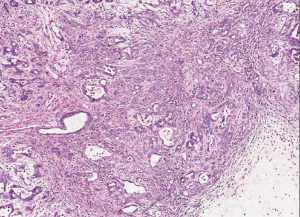
Most ductal adenocarcinoma produce an intense desmoplastic reaction that consists of collagen, myofibroblasts, lymphocytes, and other inflammatory cells. The desmoplastic reaction is responsible for the firm consistency that is grossly encountered. Infiltrating ductal adenocarcinoma can extend into the ducts and can mimic a PanIN. Most of infiltrating adenocarcinoma infiltrate and grow along nerves. Perineurial invasion is seen in over 75 percent of cases (3). In addition, ductal adenocarcinoma commonly infiltrates the lymphatics. Lymphatic invasion is associated with lymph node metastasis.
Frozen section evaluation
Frozen section can be extremely challenging. Invasive well-differentiated ductal carcinoma can look bland, and sometimes reactive glands seen in chronic pancreatitis can mimic an invasive cancer. The most useful histologic features of infiltrating adenocarcinoma on frozen section include: a haphazard pattern of growth, finding glands immediately adjacent to a muscular vessel without intervening stroma or acini, perineural or vascular invasion, finding that the area of one nucleus is four or more times larger than the area of another nucleus within a single gland, irregular nucleoli, necrotic glandular debris and the presence of numerous mitotic figures.
Immunohistochemistry
Most ductal adenocarcinoma of the pancreas express the cytokeratins (CKs) 7, 8, 13, 18, and 19. CK 20 is expressed in less than 20 percent. Ductal adenocarcinoma also expresses a variety of glycoprotein tumor antigens including CEA, CA19-9, B72.3, CA125 and DUPAN-2. The expression of CEA, B72.3, and CA125 may be useful in distinguishing infiltrating adenocarcinoma or high-grade PanIN from reactive glands. Ductal adenocarcinoma also expresses several high molecular weight glycoproteins including MUC1, MUC3, MUC4 and MUC5AC. The majority of IPMNs and mucinous cystic neoplasms (MCNs) express MUC2 but not MUC1.
Cytology
Initial approach to cytology specimen from pancreas begins with low power examination of the cellularity, cellular composition, architectural pattern, cohesiveness of the cells, and the presence of inflammation, mucin, and necrosis. High cellularity is not as important criterion for poorly differentiated carcinoma as it is for well-differentiated carcinoma. Smears of carcinoma should be relatively pure with ductal cells. Smears composed of both acinar and ductal cells should be interpreted with caution. The presence of granulation tissue and fibrous tissue fragments with inflammation is associated more with active pancreatitis than carcinoma. Carcinoma cells are arranged in crowded sheets, three-dimensional clusters or balls, to single cells. Arrangement in groups and sheets is seen more often in well-differentiated carcinoma and single intact cells are common in poorly differentiated carcinoma. In poorly differentiated carcinomas, the cellular features of malignancy are overt. The cells have a high nuclear to cytoplasmic ratio and this yields an increased nuclear density more than that seen in the benign ductal groups.
Molecular genetics
Germline mutations in genes of the Fanconi anemia pathway, which encode proteins involved in the repair of DNA cross-linking damage, have been strongly associated with familial PDA. Germline mutations in BRCA2, a crucial component of this pathway, result in increased risk of breast, ovarian, pancreatic, and other cancers (4). KRAS is the most frequently altered oncogene in PDA (somatic KRAS mutations are present in >90% of cancers), clearly indicating that this gene is a driver of tumorigenesis in the pancreas. Several frequently altered tumor suppressor genes have also been identified in PDA, including p16/CDKN2A, TP53, and SMAD4/DPC4, and these genes also represent drivers in pancreatic tumorigenesis (5). p16/CDKN2A is the most frequently altered tumor suppressor gene in ductal adenocarcinoma, with loss of p16 protein function identified in more than 90% of carcinomas. KRAS mutations are identified in more than 90% of low-grade PanINs, suggesting that KRAS mutations may represent a key initiating step in pancreatic neoplasia. In contrast, loss of Smad4 and TP53 mutation are late events, occurring only in high-grade PanIN and invasive PDA.
The gene PALB2 (also known as FANCN) encodes a protein that interacts with the BRCA2 protein. Germline mutations in PALB2 account for a subset (~3%) of patients with familial PDA. Germline mutations in p16/CDKN2A cause familial atypical mole melanoma syndrome (FAMMM); patients with this syndrome have an increased risk of melanoma (with multiple nevi and atypical nevi) and PDA. Germline mutations in STK11/LKB1 are associated with PJS, a syndrome associated with gastrointestinal hamartomas and pigmented macules on the lips and buccal mucosa, as well as cancer predisposition, including an increased risk of PDA. PDAs in these patients show somatic loss of the wild-type STK11/LKB1 allele, indicating the importance of bi allelic inactivation of the gene for carcinoma development in these patients.
Patients with hereditary pancreatitis are also at markedly increased risk for PDA. Hereditary pancreatitis, caused by germline mutations in PRSS1 and SPINK1, is characterized by young age at onset and continuous or relapsing chronic pancreatic inflammatory disease. In contrast to patients with germline mutations in tumor suppressor genes, the increased risk of cancer in these patients appears to be the result of repeated bouts of inflammation and repair, and the increased cancer risk is limited to the pancreas. Hereditary nonpolyposis colorectal cancer (HNPCC or Lynch syndrome) is caused by germline mutations in hMSH2, hMLH1, hPMS1, hPMS2 or hMSH6 (formerly GTBP), which lead to defects in DNA mismatch repair (and thus microsatellite instability), as well as markedly increased risk of carcinomas of the colon and other sites. There appears to be a slight, but real, increased risk of PDA in patients with HNPCC.
Compared with PDA, colloid carcinoma (mucinous noncystic carcinoma) has a better prognosis, a lower prevalence of KRAS mutations (approximately 30%) and TP53 mutations (approximately 20%), and a high prevalence of somatic mutations in GNAS, an oncogene that is frequently altered in IPMNs and their associated carcinomas. Medullary carcinoma, another variant with a better prognosis than ductal adenocarcinoma, has a high prevalence of microsatellite instability and lacks somatic mutations in KRAS, although oncogenic BRAF mutations have been reported. Undifferentiated carcinoma is an aggressive neoplasm with a poor prognosis; in addition to frequent KRAS mutations, these carcinomas exhibit frequent loss of E-cadherin protein expression, which provides a possible explanation for the carcinoma’s discohesive morphology. Adenosquamous carcinoma has molecular features similar to ductal adenocarcinoma, showing frequent alterations in KRAS, p16/CDKN2A, SMAD4/DPC4, and TP53, but it is an aggressive neoplasm with poor prognosis.
Prognostic markers
The prognosis of pancreatic cancer patients is very poor, with a 5-year survival of less than 6%. RTK-like orphan receptor 2 (ROR2) is overexpressed in several cancers and has increased tumorigenic activity. The expression of ROR2 and its prognostic significance have been evaluated in several studies. ROR2 plays an essential role as a prognostic marker of survival in patients with PDA and could be considered a potential therapeutic target (6). Cancer associated fibroblasts (CAFs) are a type of cell population that has an influence on tumor initiation and progression. CD146 is a cell membrane protein that is expressed in multiple cancers and also detected in PDA stromal cells. CD146 expression in CAFs is reduced by interaction with cancer cells. This finding suggests that decreased CD146 expression in CAFs promotes pancreatic cancer progression. CDKN2A methylation is correlated with an increased risk of pancreatic cancer. CDKN2A methylation plays a critical role in pancreatic carcinogenesis and may serve as a prognostic marker.
Positive Nectin-2 and DDX3 expression were associated with the progression and poor prognosis in pancreatic adenocarcinoma patients (7). Nectin-2 and DDX3 expression was significantly higher in pancreatic adenocarcinoma tumors than in peritumoral tissues, benign pancreatic tissues, and normal pancreatic tissues. Nectin-2 and DDX3 expression was significantly lower in PDA patients without lymph node metastasis and invasion and having TNM stage I/II disease than in patients with lymph node metastasis, invasion, and TNM stage III/IV disease. The ezrin protein played an important role in the progression of PDAC, and the overexpression of ezrin protein might be a useful prognostic marker of PDAC (8). B2M and loss of ALK7 expression have also been associated with invasion, metastasis, and poor-prognosis of the PDA (9).
Mucinous cystic neoplasms (MCNs)
MCN are composed of epithelial cells that produce mucin and are associated with an ovarian type stroma. The neoplastic cells form cysts contain mucoid fluid. They can be classified as follows: MCN with low grade, moderate and high grade dysplasia based on the architectural and cytologic atypia. MCN almost exclusively occur in women. If there is an associated invasive component, the lesion is designated as MCN with an associated carcinoma.
Pathologic features
Gross
The majority of MCN occur in the body or tail of the pancreas. MCN can be large measuring up to 36 cm. The cut surface can be unilocular or multilocular, with cysts ranging from a few millimeters to several centimeters in diameter. The cysts content consist of thick mucin or mucin and hemorrhagic material. Higher grade neoplasms usually have papillary excrescences.
Histopathology
The two most prominent components identified in MCN include the epithelial lining and an ovarian type stroma (Figure 2). The cysts are lined by columnar, mucin-producing epithelium that stain with diastase-resistant periodic acid-Schiff (PAS) and Alcian blue. The columnar cells have abundant intracytoplasmic apical mucin and can form papillae. The cysts usually do not communicate with the pancreatic ducts. In MCN with low grade dysplasia, the columnar epithelium has only minimal architectural and cytological atypia and mitosis are absent. MCN with moderate dysplasia have moderate architectural and cytological atypia with papillary projections or crypt-like invaginations. MCNs with high-grade dysplasia have significant architectural and cytological atypia with papillae with irregular and complex branching, nuclear stratification, loss of polarity and pleomorphism. Approximately up to one third of MCNs have an associated invasive carcinoma.
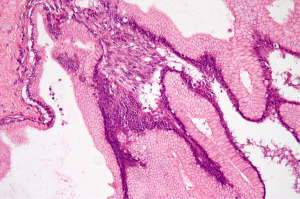
Immunohistochemistry
The epithelial cells in MCN are positive for CKs 7, 8, 18 and 19; CAM5.2; and CEA. It is also positive for MUC5AC, MUC2 and pancreatic type mucin markers DUPAN-2 and CA 19-9. The ovarian type stroma is positive for vimentin, smooth muscle actin, desmin and calretinin.
Molecular genetics
Activating point mutations in codon 12 of the KRAS gene appear early and increase in frequency proportional with the degree of dysplasia. Inactivation of the SMAD4/DPC4 tumor suppressor gene also appears to be a late event. Alterations of TP53, CDKN2 (P16) tumor suppressor genes are more frequent in cases associated with an invasive component.
Intraductal papillary mucinous neoplasm (IPMN)
IPMN are the most common and best characterized of the intraductal neoplasms. Noninvasive IMPNs are categorized into three groups based on the degree of architectural and cytological dysplasia: IPMN with low grade dysplasia, IPMN with moderate dysplasia, and IPMN with high grade dysplasia (or intraductal papillary mucinous carcinoma in situ). IPMN grows within the main pancreatic duct or one of its branches and most often has a papillary architecture.
Pathologic features
Gross
The majority of IPMNs arise in the head of the pancreas. On sectioning, the main pancreatic duct or one of the branches is usually dilated. The duct is often filled with mucin and is tortous and irregular. The documentation of involvement of the main pancreatic duct is important because this is associated with a higher risk of high grade dysplasia and invasive carcinoma. The involved pancreatic parenchyma is often firm, reflecting changes of chronic pancreatitis.
Histopathology
IPMN are characterized by the intraductal proliferation of mucin-producing cells (Figure 3). IPMNs lack the ovarian type stroma usually seen in MCNs. The neoplastic cells conform to the branching of the pancreatic duct system, confirming the intraductal growth of these neoplasms. The size of the papillary formations ranges from subtle microscopic projections to grossly visible papillae measuring up to several centimeters in length. IPMN with high grade dysplasia has a severe architectural complexity and nuclear atypia with cribriforming and budding off clusters of neoplastic cells to the lumen of the duct.
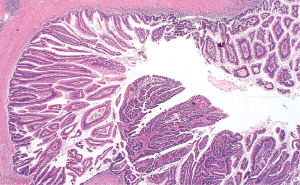
Immunohistochemistry
IPMN are positive for CKs 7 and 19, CA 19-9, B72.3 and CEA. Most label pancytokeratin antibodies (AE1/AE3) as well as CAM5.2.
Molecular genetics
Activating point mutations in codons 12 or 13 of the KRAS oncogene has been reported. In most cases the frequency of KRAS gene mutations increases with the increasing grade of dysplasia. PIK3CA gene mutations have been reported in about 10 percent of IPMNs. Allelic losses involving loci of tumor suppressor genes, including CDKN2A, TP53, and SMAD4, are found in up to 40 percent of IPMNs, and these increases with increasing degree of dysplasia (10).
Pancreatic neuroendocrine neoplasms
Endocrine neoplasms of the pancreas are neoplasm with a predominantly neuroendocrine differentiation. Most are well-differentiated and low grade neoplasms. They are classified as well-differentiated (low- intermediate grade) and poorly differentiated (high grade) neuroendocrine carcinoma.
Pathologic features
Gross
About 60 percent of neuroendocrine tumors arise in the tail of the pancreas. They are usually circumscribed or encapsulated and are composed of red to yellow parenchyma. They can be soft and fleshy or densely fibrotic. Areas of hemorrhage or necrosis can occur, usually in larger neoplasms. Neuroendocrine carcinoma is firm white-grey masses with ill-defined borders and often exhibit areas of necrosis and hemorrhage.
Histopathology
Pancreatic neuroendocrine tumors have an organoid histological pattern, characterized by nesting, trabecular, glandular or pseudorosette arrangements. The cells are uniform, with amphophilic cytoplasm and a centrally located round nucleus. The chromatin pattern is coarsely clumped “salt and pepper” (Figure 4). In many cases mitoses are undetectable, but generally by definition neuroendocrine tumors have less than 20 mitoses per 10 HPF. Neuroendocrine carcinoma by definition have more than 20 mitoses per HPF. Most cases have necrosis.
Molecular genetics
Little is known about the molecular basis for sporadic tumors. Activation of oncogenes is not a common event in pancreatic neuroendocrine tumors. Losses of 11q13 seen in reported cases indicate the possibility that a yet unknown tumor suppressor gene might be associated.
Solid-pseudopapillary neoplasm of the pancreas
Solid-pseudopapillary neoplasm of the pancreas is a low grade malignant epithelial neoplasm. Is composed of poorly cohesive polygonal epithelial cells forming solid and pseudopapillary structures. The neoplastic cells are composed of uniform nuclei and nuclear grooves. It occurs predominantly in women.
Pathologic features
Gross
Solid-pseudopapillary neoplasms are relatively distributed throughout the pancreas. Most neoplasms are large, with a mean diameter of 10 cm. On cut surface the tumor is soft, white-gray to yellow solid areas. Approximately 90 percent are solid and cystic. Areas of necrosis, hemorrhage and cystic degeneration is common.
Histopathology
The microscopic pattern is heterogeneous, with solid, pseudopapillary, hemorrhagic and necrotic areas. Solid areas are poorly cohesive monomorphic cells that are admixed with myxoid stroma and thin walled blood vessels. Pseudopapillae are formed when poorly cohesive cells drop out, leaving a variable aggregates of loosely cohesive cells between fibrovascular stalks (Figure 5). The cytoplasm is usually eosinophilic or clear, but can also be foamy. The nucleus is round to oval and uniform with longitudinal nuclear grooves (Figure 5).
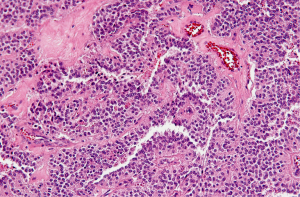
Immunohistochemistry
The majority of solid-pseudopapillary neoplasms express alpha-1-antitrypsin, NSE, vimentin, progesterone receptors, CD10, CD56, galectin 3, cyclin D1 and beta-catenin.
Molecular genetics
Approximately 90 percent of solid-pseudopapillary tumors have somatic point mutations in exon 3 of the beta-catenin gene.
Acinar cell carcinoma
Acinar cell carcinoma of the pancreas is an uncommon tumor, it accounts for less than 2 percent of all pancreatic carcinomas (11). Occurs more often in elderly patients in the seventh and eight decade of life, and there is a male predominance
Pathologic features
Gross
Acinar cell carcinoma are usually large, solid and well circumscribed tumors that often show necrosis and cystic degeneration. They have a soft consistency, often due to a lack of prominent stromal component.
Histopathology
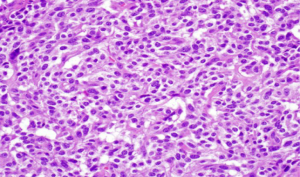
Acinar cell carcinoma is usually highly cellular tumors and often lacks a desmoplastic response that is often characteristic of ductal adenocarcinoma. The tumor is composed of solid sheets or nests of cells that are monotonous and small glandular spaces. The cells have abundant eosinophilic and granular cytoplasm due to zymogens granules (Figure 6). The nuclei usually have a single and prominent nucleoli and often there is increased mitotic activity. Zymogen granules usually stain positive with PAS staining.
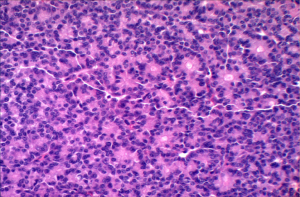
Immunohistochemistry
BCL10 has been a sensitive immunohistochemical marker. Staining for trypsin, lipase, and chymotrypsin is usually positive.
Molecular genetics
Acinar cell carcinoma lacks abnormalities in KRAS, TP53, CDKN2A and SMAD4 genes. However, mutations in the APC/beta-catenin pathway occurs in approximately 25 percent of acinar cell carcinomas (12). Allelic losses of chromosome 11 percent are identified in 50 percent of cases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (R. Charles Nichols Jr, Debashish Bose and George P. Kim) for the series “Pancreatic Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.12.01). The series “Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ahlgren JD. Epidemiology and risk factors in pancreatic cancer. Semin Oncol 1996;23:241-50. [PubMed]
- Kumar V, Robbins SL. Robbins basic pathology. 8th ed. Philadelphia: Saunders/Elsevier, 2007.
- Bosman FT, Carneiro F, Hruban RH, et al. WHO Classi fication of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer (IARC) Press, 2010:279-337.
- Lal G, Liu G, Schmocker B, et al. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Res 2000;60:409-16. [PubMed]
- Couch FJ, Johnson MR, Rabe K, et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res 2005;65:383-6. [PubMed]
- Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003;8:645-54. [PubMed]
- Liang S, Yang Z, Li D, et al. The Clinical and Pathological Significance of Nectin-2 and DDX3 Expression in Pancreatic Ductal Adenocarcinomas. Dis Markers 2015;2015:379568. [Epub ahead of print].
- Piao J, Liu S, Xu Y, et al. Ezrin protein overexpression predicts the poor prognosis of pancreatic ductal adenocarcinomas. Exp Mol Pathol 2015;98:1-6. [PubMed]
- Liu C, Yang Z, Li D, et al. Overexpression of B2M and loss of ALK7 expression are associated with invasion, metastasis, and poor-prognosis of the pancreatic ductal adenocarcinoma. Cancer Biomark 2015;15:735-43. [PubMed]
- Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas (Atlas of Tumor Pathology Series 4). Washington: American Registry of Pathology; 2007:388.
- Klimstra DS, Klöppel G. Tumors of the exocrine pancreas. In: Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 4th ed. Philadelphia: Saunders;2013:531-88.
- Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815-37. [PubMed]


