
Irradiation and combination immunotherapy
Our ability to control and eradicate cancer has advanced with new combinations of surgery, chemotherapy, irradiation therapy and recently, immunotherapy. The concept of a single cancer therapy has passed with each new discovery revealing the complexity of the genetics and immunology of the tumor microenvironment. Understanding which combination of standard and emerging therapies that can provide long lasting remission of each particular cancer is paramount. Here we review the capacity of radiation therapy (RT) combined with immune checkpoint inhibitors to induce a complete response in mammary carcinoma and melanoma (1,2) and the evaluation of therapy mechanism of action using intravital microscopy. Current tools and approaches to evaluate mechanism of action of therapies have been limited, but real time intravital imaging offers prospects of enhancing our knowledge.
One standard of care cancer therapy of immunological interest is RT. Local RT is effective at killing tumor cells directly, but the effect of RT can extend beyond the treated primary tumor. The abscopal effect of RT is an anti-tumor immune response generated at sites distant (systemic) from the irradiated volume (3). Immunogenic tumor cell death caused by RT represents in effect an in situ vaccine specific for that patient (4). It provides neighboring antigen presenting cells with tumor antigens, neoantigens (non-self peptides that are generated by the mutated cancer genome), and activating danger signals, such as HMGB1 which binds to TLR4, and calreticulin which leads to priming and activation of tumor-specific T cells capable of attacking the tumor at primary or distant sites (5). RT also increases the T cell receptor (TCR) repertoire, which allows the expansion of T cells clones against the tumor with diverse TCR traits (2). Studying this process has been limited to in vitro studies and “snap shot” analysis of mouse tumors and perturbed immune systems exposed to RT. The application of imaging technologies to study pathology is one of the most transformative advancements in medicine and as technology advances, it will continue to have broader applications in the future (Figure 1).
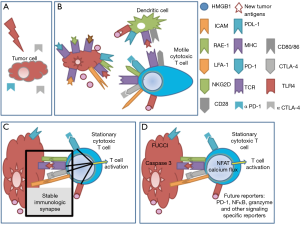
Previously, it has been shown via single cell microscopy that stable immunological synapse formation between DCs and T cells is required for signalling and that productive interactions can be determined by the prolonged time of contact of the membranes of each cell (6). The outcome of that interaction, whether activating or suppressing, requires the use of specific reporters or other assays. The engagement of CTLA-4 on T cells by CD80/CD86 on DC is a negative regulatory signal for T cells. Immune checkpoint antibodies, like anti-CTLA-4 and anti-PD-1, work by blocking the mechanisms that hinder the activation and function of anti-tumor T cells. Through intravital imaging, anti-CTLA-4 has been shown to increase T cell motility and reduce contact periods between T cells and antigen-presenting cells (7).
Two photon intravital imaging represents a more contemporary way to study the effects of RT and anti-CTLA-4 combination therapy. Ruocco et al. studied RT in combination with anti-CTLA-4 (9H10) treatment in a non-immunogenic mouse mammary cancer model (4T1) (1). They showed that standard of care ionizing RT, is able to induce immunogenic tumor antigens and other microenvironment changes required for a robust anti-tumor response. Intravital microscopy was used to determine the efficacy of immunotherapy on tumor growth, describing the direct interaction of CXCR6+ CD8+ T cells with the CFP+ tumor and the behaviour of the CD8+ T cells within the tumor microenvironment after RT and/or anti-CTLA-4 therapy. RT and anti-CTLA-4 therapy in combination was shown to control the growth of established tumor and this was attributed to the enhanced infiltration of activated CD8+ T cells (1).
Ruocco et al. (1) also showed that anti-CTLA-4 antibody treatment increased T cell motility in the tumor microenvironment, whereas anti-CTLA-4 treatment with RT promoted T cell arrest in contact with tumor cells. This T cell interaction with tumor cells was an MHC class I-dependent antigen-specific event. Anti-CTLA-4 treatment increased T cell motility on ICAM-1-coated surfaces. After RT, 4T1 cells upregulated expression of MHC class I, ICAM-1, and the NKG2D ligand, RAE-1γ. By using an NKG2D blocking antibody, DX5, with RT and anti-CTLA-4 treatment they showed that the T cell-tumor interactions were decreased and T cell velocity increased suggesting that NKG2D plays a role in stable interactions between CD8+ effector T cells and tumor cells. Although NKG2D does not play a role in RT-reduced primary tumor growth, the upregulation of RAE-1 does play a role in primary tumor growth in the context of RT and anti-CTLA-4 therapy, which was also shown to hold true in the experimental metastatic model. Taken together this suggests that tumor antigen recognition by the TCR of CD8+ effector T cells after RT is stabilized by NKG2D-RAE-1 interactions and activation is enhanced by anti-CTLA-4 treatment resulting in tumor control.
In concert with these combination benefits of RT and immune checkpoint blockade immunotherapy, a subsequent study showed that RT in combination with anti-CTLA-4 had an 18% partial as best response in humans and 17% response in mice with melanoma (2). Additionally, PD-1 is a negative regulatory signal for T cells, where blocking its interaction with PDL-1 on antigen presenting cells or tumor cells has had profound therapeutic effects especially in melanoma patients resulting in an increase in activated T cells (8). Remarkably when RT and anti-CTLA-4 were combined with anti-PD-1 therapy, complete response rates in mice increased to 80% (2). Indeed, the appeal of immune checkpoint blockade therapy is that it induces long lasting anti-tumor responses in patients with advanced-stage cancers.
Intravital microscopy has been used to study the dynamic in vivo immune cell responses to infection, autoimmunity and cancer (9,10). Many of the initial tumor intravital studies focused on the development of angiogenesis and the efficacy of anti-angiogenic therapies using intravascular injection of fluorescent dyes (11). Vessel response to RT in a dorsal skin fold chamber showed that there was capillary constriction and thrombus formation from day 4 up to 20 days after treatment (12). Recently this technology has been used to determine the efficacy of a therapy, from chemotherapy penetrance to tumor apoptosis (13,14). There has been an increasing interest in immune cell interactions with tumors and other cells within the tumor microenvironment following the burgeoning field of immunotherapy. Together with development of fluorescent reporter mice to distinguish immune cell subsets and fluorescent reporter tumor cell lines, the migration, invasion and metastasis of tumors have described unexpected interactions with vessels, ECM and the bone marrow niche (9,10,15). The greatest benefit of intravital imaging is the ability to assess the early development of the tumor and interactions between small numbers of transformed cells and immune cells. A consideration of this technique is the depth of penetration where in some cases the first 150 µm of the 400 µm from the outside of the tumor is encapsulation, therefore in a heterogeneous tumor population with potentially a hypoxic or necrotic core, it is important to confirm findings using other methods such as immunofluorescent imaging of tissue sections.
The next important advancement in intravital imaging is real time signalling reporters of immune cell interactions that can be used to predict efficacy of therapies, either by reporting the signalling in immune cells, the metabolic state of cells within the microenvironment, or the apoptosis of tumor cells (14). The NFAT reporter was developed to allow the visualization of activated T cells and can be used to determine the percentage of activated cytotoxic cells within the tumor microenvironment (16). FRET reporters of calcium flux used in neuroscience have also been used to show TCR signalling and recognition of cognate antigen (17). This calcium reporter can be useful in the context of RT and immunotherapy to quantify the number of antigen-specific cytotoxic CD8+ T cells at the tumor site after therapy. The use of a FRET capspase-3 reporter allows the visualization of apoptotic tumor cells (18), but if multiplexed with additional information about other cells in the tumor microenvironment it could prove to be a powerful tool in dissecting the mechanism of action [reviewed in (14)]. Inhibiting cancer stem cells is a therapeutic approach of interest (15). The Confetti fluorescent construct which randomly assigns different colours to individual cells (19) is useful for lineage tracing of cancer stem cells and has shown that certain clones outcompete adjacent tumor cells. The FUCCI reporter construct allows the visualization of the different stages of cell cycle and is useful in determining whether a therapy is able to stop caaancer cell proliferation and the point of cell cycle can be determined (20). Although classical immunological assays allow us to determine the global efficacy of therapies, intravital imaging and the new reporter constructs are unique in resolution of space and time in providing insights into complex interactions within the tumor microenvironment (Figure 1D). Ruocco et al. effectively used intravital imaging to show that antigen specific recognition of tumor cells after RT is stabilized by NKG2D-RAE-1 interactions resulting in tumor control. The future of intravital imaging is in the development of new functional fluorescent reporters, specific to critical signalling pathways for the direct analysis of therapy mechanisms.
Acknowledgments
The authors thank Dr. Shin Foong Ngiow for his helpful discussions.
Funding: The authors were supported by a National Health and Medical Research Council (NHMRC) of Australia Program Grant (1013667) and Research Fellowship (1078671 to MJS) and an Australian Cancer Research Foundation (ACRF) Equipment grant.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest 2012;122:3718-30. [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [PubMed]
- Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015;356:82-90. [PubMed]
- Vanpouille-Box C, Pilones KA, Wennerberg E, et al. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 2015;33:7415-22. [PubMed]
- Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74-88. [PubMed]
- Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol 2008;8:675-84. [PubMed]
- Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, et al. Cytotoxic T lymphocyte antigen-4 blockade enhances antitumor immunity by stimulating melanoma-specific T-cell motility. Cancer Immunol Res 2014;2:970-80. [PubMed]
- Homet Moreno B, Parisi G, Robert L, et al. Anti-PD-1 therapy in melanoma. Semin Oncol 2015;42:466-73. [PubMed]
- Beerling E, Ritsma L, Vrisekoop N, et al. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci 2011;124:299-310. [PubMed]
- Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer 2003;3:921-30. [PubMed]
- Fukumura D, Duda DG, Munn LL, et al. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 2010;17:206-25. [PubMed]
- Maeda A, Leung MK, Conroy L, et al. In vivo optical imaging of tumor and microvascular response to ionizing radiation. PLoS One 2012;7:e42133 [PubMed]
- Giedt RJ, Koch PD, Weissleder R. Single cell analysis of drug distribution by intravital imaging. PLoS One 2013;8:e60988 [PubMed]
- Ellenbroek SI, van Rheenen J. Imaging hallmarks of cancer in living mice. Nat Rev Cancer 2014;14:406-18. [PubMed]
- Hart LS, El-Deiry WS. Invincible, but not invisible: imaging approaches toward in vivo detection of cancer stem cells. J Clin Oncol 2008;26:2901-10. [PubMed]
- Marangoni F, Murooka TT, Manzo T, et al. The transcription factor NFAT exhibits signal memory during serial T cell interactions with antigen-presenting cells. Immunity 2013;38:237-49. [PubMed]
- Direnberger S, Mues M, Micale V, et al. Biocompatibility of a genetically encoded calcium indicator in a transgenic mouse model. Nat Commun 2012;3:1031. [PubMed]
- Keese M, Yagublu V, Schwenke K, et al. Fluorescence lifetime imaging microscopy of chemotherapy-induced apoptosis resistance in a syngenic mouse tumor model. Int J Cancer 2010;126:104-13. [PubMed]
- Zomer A, Ellenbroek SI, Ritsma L, et al. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells 2013;31:602-6. [PubMed]
- Janssen A, Beerling E, Medema R, et al. Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS One 2013;8:e64029 [PubMed]


