
Skipping the line: bringing MET exon 14 skipping mutations to the forefront of targeted therapy
Our understanding of the oncogenic role and targetability of the mesenchymal-to-epithelial transition (MET) oncogene in non-small cell lung cancer (NSCLC) has made remarkable strides in the last year through the discovery of recurrent, actionable MET exon 14 skipping alterations in NSCLC. The MET gene, located at chromosome 7, encodes for the hepatocyte growth factor (HGF) receptor, a tyrosine kinase receptor important for cell proliferation, apoptosis and motility/invasion and has long been believed to be a potentially relevant oncogene in certain settings. In fact, recurrent MET gene mutations had been reported in certain types of papillary renal cell cancer, including familial cases, and sporadic MET mutations have been previously reported in NSCLC, however until recently, targeting MET in NSCLC has been fraught with challenges (1). Several groups have now reported their observations of recurrent, actionable MET exon 14 alterations, generating greater interest and improved clarity in the potential incorporation of MET testing and MET targeting into current treatment algorithms in this age of personalized medicine. We will hereby review the recent important study of Awad and colleagues in the context of other recent discoveries dramatically changing the diagnostic and treatment landscape in this field.
MET targeting for NSCLC has initially been met with setbacks, highlighting the difficulty in identifying a reliable biomarker for MET inhibitor activity. The anti-MET monoclonal antibody onartuzumab was used in combination with erlotinib in a phase II trial showing a trend towards an improved PFS and OS in those patients demonstrating MET IHC positivity (2). This observation led to the METLung trial, a phase III study, focused on those patients with MET IHC 2+ or 3+ in >50% tumor cells, but was unfortunately closed prematurely due to lack of clinical benefit (3). Tivantinib, a non-ATP-competitive small molecular inhibitor of MET, was initially examined in a phase II trial comparing erlotinib and tivantinib with erlotinib and placebo in unselected advanced non-squamous NSCLC patients with initial trends showing a benefit for MET IHC positive patients leading to the MARQUEE trial. This phase III trial, unfortunately, was also closed early due to futility (4). The use of crizotinib, a dual MET/ALK inhibitor, was first reported to show some efficacy in a Phase I study presented by Camidge and colleagues at the American Society of Clinical Oncology (ASCO) 2014 Annual Meeting (5). Patients with advanced NSCLC were categorized by their MET amplification status as either low and intermediate or high as determined by FISH, and then treated with crizotinib. They observed that as MET amplification increased, an increased percent of objective partial responses was observed.
Activation of MET can be mediated by gene amplification, protein overexpression, point mutations and also alternative splicing leading to exon 14 skipping (6). Exon 14 of MET encodes the juxtamembrane region which contains key regulatory elements including Y1003, the direct binding site for Cbl, an E3 ubiquitin ligase, that promotes c-Met protein degradation (7). Exon 14 skipping results in loss of Y1003, leading to decreased MET ubiquitination, resulting in increased MET levels (8). Several studies now have identified a special subset of patients with MET exon 14 skipping in NSCLC, supporting its role in oncogenesis. The reported frequencies of MET exon 14 skipping mutations in lung adenocarcinomas consistently range in the 3–4% range (Table 1) and a uniquely aggressive subtype of lung cancer, so-called sarcomatoid lung cancer has been reported to harbor a much higher, up to 25–35%, frequency of these mutations (9-13).
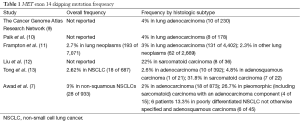
Full table
Several groups have also reported on selected patients with exon 14 skipped tumors treated with MET inhibitors with most cases demonstrating at least a partial response. Collectively, the number of patients with MET exon 14 alterations that have been reported and treated with MET inhibitors are limited to small case series, however, the aggregate has shown significant promise and sparked renewed interest in MET inhibition for NSCLC leading to the actual incorporation of testing and MET inhibitor therapy recommendation for exon 14 skipped patients in the NCCN guidelines. Table 2 lists the reported cases thus far, all of which have demonstrated at least a partial/clinical response to a MET inhibitor (7,10-12,14-17).
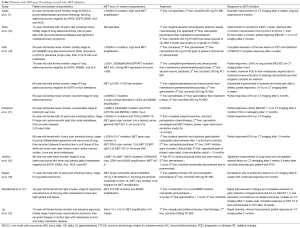
Full table
In the current manuscript, Awad and colleagues report on the comprehensive analysis of 6,376 cancers, using next generation sequencing (NGS) encompassing the MET gene which included 1,141 lung cancers between August 1, 2013 and May 1, 2015 (7). Twenty-eight patients of 933 non-squamous NSCLC (3%) harbored exon 14 mutations, consistent with frequencies previously reported such as in the TCGA database as well other studies (9). Eighteen of the patients had adenocarcinoma, four had pleomorphic (i.e., sarcomatoid) carcinoma with an adenocarcinoma component, five had poorly differentiated NSCLC not otherwise specified, and one had adenosquamous histology. The group made several interesting observations from their cohort. Of note, all 28 patients were white, non-Hispanic. The median age at disease onset was 72.5 years. They found that patients with MET exon 14 mutations were significantly older than patients with EGFR mutations (P<0.001) and KRAS mutations (P<0.001) during the same time period. They also found that 64% of patients with MET exon 14 mutations had a smoking history.
Of the 28 patients, several different mutation types in MET exon 14 and its flanking introns were observed. Genomic deletions were identified in 17 (61%) patients and point mutations in the remaining 11 (39%) patients. A qRT-PCR-based assay on 24 samples with adequate RNA material confirmed exon 14 skipping in 23 samples (96%) showing that the various sequence changes can indeed similarly affect precursor mRNA processing. No concurrent genomic alterations in KRAS, EGFR, ERBB2, ALK, ROS1 or RET were identified in the 28 patients suggestive of these being mutually exclusive, driver oncogene events. Analysis of genomic copy number changes showed that 6 tumors (21%) had high-level MET copy gain and 8 (29%) had low-level MET copy gain. Twenty-five patients had sufficient tissue for immunohistochemical c-MET expression analysis which ranged from weak to maximum expression demonstrating poor concordance questioning the utility of MET IHC in this setting. They also observed that patients with stage IV disease had a significantly higher H score with a mean of 253 than patients with stage I to III disease (P=0.002) and stage IV NSCLCs without MET exon 14 mutations (P<0.001).
Adding to the growing list of patients with MET exon 14 mutations reported in the literature treated by MET inhibitors, Awad and colleagues also reported on a patient with stage IV NSCLC with poorly differentiated carcinoma, favoring adenocarcinoma, with MET exon 14 skipping and high-level MET amplification, who was treated with crizotinib. After 8 weeks, repeat imaging demonstrated a major partial response consistent with a very high likelihood of treatment response highlighted by prior cases as well.
As we continue to see reports of remarkable efficacy with MET inhibitors in patients with MET exon 14 mutations, clearly more widespread studies to generate a treatment algorithm need to be designed, however even the current limited information strongly argues for a trial of MET inhibitor therapy on or off study (such as with crizotinib or cabozantinib) in the absence of other options in metastatic disease in patients with MET exon 14 skipped, advanced NSCLC. The observed MET mutation frequencies have been on par with ALK and ROS mutations, influencing possible approaches to the way in which patients may be tested for MET exon 14 mutations. In patients with lung cancer who have tested negative for EGFR, KRAS, ALK and ROS1, adding MET to the panel of mutations to be tested should be very strongly considered and this might be best accomplished as part of multi-gene panels. In fact, the NCCN guidelines have already adopted such recommendations given the powerful observations listed without a formal study in this area.
A key component needed to streamline testing for MET mutations is the identification of a validated biomarker both for patient selection and as a predictor of response to inhibition. The identification of gene level alterations as opposed to MET expression as measured by IHC perhaps may be the missing piece in identifying such a biomarker. As the technology, feasibility and costs of NGS improve and become more accessible, NGS as the standard for the evaluation of genomic alterations may prove to be the recommended testing modality and recent technological advances now allow testing for alterations such as MET exon 14 skipping as part of ctDNA-based NGS platforms.
The older patient population with MET exon 14 mutations, as has been reported by both Awad et al. and Tong et al., represent a specific group who may benefit from improved patient selection for targeted therapy as these patients frequently present with increased comorbidities and decreased performance status which may limit their tolerability for conventional chemotherapy (13). Demonstrated benefit in this scenario was recently reported in a patient with an ECOG of 4 and significant cardio-pulmonary symptoms from stage IV NSCLC with a MET exon 14 deletion, treated with crizotinib and a remarkable response within a week including improvement of ECOG to 1 (14).
Once patients have been selected, the question then lies in the optimal approach to treatment. Experience thus far has been in a handful of previously treated patients, faced with limited remaining treatment options. Further investigation of the appropriate timing of MET inhibitor administration, the optimal choice of MET inhibitor from an expanding list of currently approved and actively studied agents and the possibility of combination therapies is sorely needed. Understanding of possible resistance mechanisms similar to other targeted agents will also be of great importance.
Although several unanswered questions regarding MET as an actionable target remain, the rapid accumulation of knowledge and the excellent results with patients treated thus far inspire tremendous optimism that a highly effective additional line of care now can be offered to our patients with documented MET exon 14 skipped mutations and rapid incorporation of broad-based molecular testing including testing for MET exon 14 skipping in diagnostic algorithm is key to allow the largest number of our patients to benefit.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Qing-Yuan Huang (Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China).
Conflicts of Interest: Dr. B Halmos has received clinical research support from Mirati, Astra-Zeneca, Boehringer-Ingelheim, Merck and served as a paid consultant for Astra-Zeneca, Boehringer-Ingelheim, Genentech and Celgene; Dr. E Shum has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol 2013;31:1089-96. [Crossref] [PubMed]
- Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105-14. [Crossref] [PubMed]
- Spigel DR, Edelman MJ, O'Byrne K, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol 2014;32:abstr 8000.
- Scagliotti GV, Novello S, Schiller JH, et al. Rationale and design of MARQUEE: a phase III, randomized, double-blind study of tivantinib plus erlotinib versus placebo plus erlotinib in previously treated patients with locally advanced or metastatic, nonsquamous, non-small-cell lung cancer. Clin Lung Cancer 2012;13:391-5. [Crossref] [PubMed]
- Camidge DR, Ou SH, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8001.
- Ma PC. MET receptor juxtamembrane exon 14 alternative spliced variant: novel cancer genomic predictive biomarker. Cancer Discov 2015;5:802-5. [Crossref] [PubMed]
- Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent met genomic amplification and c-met overexpression. J Clin Oncol 2016;34:721-30. [Crossref] [PubMed]
- Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res 2006;66:283-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842-9. [Crossref] [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Shea M, Huberman MS, Costa DB. Lazarus-type response to crizotinib in a patient with poor performance status and advanced met exon 14 skipping mutation-positive lung adenocarcinoma. J Thorac Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Jenkins RW, Oxnard GR, Elkin S, et al. Response to crizotinib in a patient with lung adenocarcinoma harboring a MET splice site mutation. Clin Lung Cancer 2015;16:e101-4. [Crossref] [PubMed]
- Waqar SN, Morgensztern D, Sehn J. MET mutation associated with responsiveness to crizotinib. J Thorac Oncol 2015;10:e29-31. [Crossref] [PubMed]
- Mendenhall MA, Goldman JW. MET-mutated NSCLC with major response to crizotinib. J Thorac Oncol 2015;10:e33-4. [Crossref] [PubMed]


