
Notch signaling and Tp53/RB1 pathway in pulmonary neuroendocrine tumorigenesis
Introduction
Lung cancer has been reported to be one of the leading causes of cancer death worldwide, and especially among various types of lung cancer, small cell lung cancer (SCLC) is the most aggressive type, showing a rapid growth and metastasis, in spite of a temporary response to chemo-radiotherapy (1). Improvements in treatment of SCLC have not been remarkable in the past decades, and the standard chemotherapy regimen of cisplatin or carboplatin plus etopside, used for the first-line treatment of SCLC, has not changed over the past four decades (2). Fundamental studies on molecular mechanisms of small cell carcinogenesis have not been fully established, and significant progresses will be anticipated, in order to explore novel therapeutic development as soon as possible. In the recent years, few studies analyzing a relatively large scale, to search for essential and critical molecules in SCLC, were reported (3-5), and the importance of various pathways, such as cell cycle regulation associated with TP53 and RB1, receptor-kinase signaling, transcriptional network, Notch signaling, and guidance molecule system, were pointed out (3-5). In a recent article reported by Meder et al. (6), they proposed that secondary SCLC, could be derived from non-small cell lung cancer (NSCLC), through loss of Notch activity, accompanied with increased achaete-scute homolog 1 (ASCL1) activity, with further additional genetic changes in Tp53 and RB1. They analyzed combined type SCLC from the view point of the Notch-ASCL1-p53-RB axis, and—to our knowledge—it was the first article to discuss comprehensively the molecular mechanisms of small cell carcinogenesis regarding these four molecules. This article is very interesting as it reports (I) SCLC could be generalized basically by changes in the Notch-ASCL1-p53-RB axis; (II) pre-acquisition of potential neuroendocrine differentiation through modulating Notch-ASCL1 balance seems to be important in the development of SCLC; and (III) there could be an alternative pathway in small cell carcinogenesis.
In the current perspective, although many issues remain to be solved for understanding the molecular mechanisms of carcinogenesis of SCLC, and making reference to the article reported by Meder et al. (6), we will discuss some of the recent insights into the mechanisms of neuroendocrine differentiation, and expand the argument on small cell carcinogenesis.
Transcriptional regulation of neuroendocrine differentiation
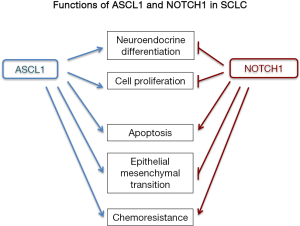
Pulmonary neuroendocrine cells are specialized epithelial cells, distributed sparsely throughout the lung epithelia, from the bronchus to the bronchio-alveolar junctional area, and could serve to maintain the homeostasis of airway microenvironments (7,8). Various transcription factors have been reported to determine neuroendocrine differentiation in the normal and neoplastic lung epithelial cells, and ASCL1; a proneural basic helix-loop-helix transcription factor, has been regarded as a neuroendocrine inducer and lineage marker (9-12). In normal epithelial cells, transfection of ASCL1 gene directed epithelial cell toward neuroendocrine differentiation, and in a lung adenocarcinoma cell line, cell morphology and proliferation activity were altered by ASCL1 transfection (6,13,14). ASCL1 appears to be involved in the cell growth, survival, differentiation, cell adhesion, and chemoresistance (Figure 1). According to Osada et al. (14),inhibition of ASCL1 suppressed cell proliferation and induced apoptosis in SCLC cell lines, which could signify that ASCL1 plays pivotal role in carcinogenesis of SCLC. Expression of ASCL1 is suppressed by Notch signaling in normal epithelial cells and cancer cells (15-17), and Hes1; one of the representative target genes of Notch signaling pathway and a repressive basic helix-loop-helix transcription factor, is a strong suppressor of ASCL1 in developing mouse lung and in SCLC cells (10,18). One of the ASCL1 candidate regulators in SCLC cells is Repressor element-1 silencing transcription factor (REST, as it suppresses the expression of ASCL1 through epigenetics mechanisms in neurogenesis (19). Besides, REST is deficient in SCLC cell lines (20).
In addition to ASCL1, Brain 2 (BRN2); a POU domain transcription factor, is a developmentally neural-cell specific factor, and could participate in neural differentiation of SCLC cells (18,21). Recently, a zinc-finger transcription factor; insulinoma-associated protein 1 (INSM1), was reported as a crucial regulator for neuroendocrine differentiation for normal lung epithelial cells (22) and SCLC cells (18), and INSM1 could regulate the expression of both ASCL1 and BRN2 in lung cancer cell lines (18). In addition, INSM1 alone can induce neuroendocrine differentiation in NSCLC cell lines (18). Moreover, the expression of INSM1 and ASCL1 was suppressed by the activation of Notch signaling (18).
Another transcription factor that regulates neuroendocrine differentiation in lung cancer cells, is retinoblastoma (RB) gene product. There is an interesting report, which showed that increased pulmonary neuroendocrine cells are observed in Rb1 gene-deficient mouse lungs (23). Considering that RB1 is one of the essential genetic abnormalities in SCLC, an attractive molecular research field for studying the relation between neuroendocrine differentiation and RB abnormalities remains to be explored. In addition, pulmonary neuroendocrine cell hyperplasia in Rb1 gene-deficient mice disappears with loss of of E2f3, one of Rb1 targets (24).
Significance of Notch1 in small cell carcinoma
Notch signaling is one of the most important cell signaling system, and through interaction with ligands of the Delta and/or Jagged/Serrate families, it regulates several genes such as Hes1, cyclinD1, c-Myc and Akt (25). The importance of Notch signaling in carcinogenesis has been reported in controlling the differentiation, metabolism, cell cycle progression, angiogenesis, stemness, and of cancer cells (26). In lung cancer, Notch exhibits both tumor promoting and suppressive functions. A whole genome sequencing study of SCLC cases revealed mutations of Notch family genes in about 25% of the cases examined, suggesting a tumor suppressive nature of Notch in SCLC cells (5). In SCLC cell lines, gene transfection and knockdown experiments clarified that Notch1 plays significant role in suppression of cell proliferation, enhancement of apoptosis, induction of epithelial morphology (mesenchymal-epithelial transition), suppression of motility, acquisition of drug resistance and suppression of neuroendocrine differentiation (Figure 1) (17,27-29). Regarding cell fate determination, Notch1-Hes1 pathway is a repressor of neuroendocrine differentiation through decreased expression of ASCL1 and INSM1 (10,16,18,30). Using immunohistochemistry, pulmonary neuroendocrine cells are positive for Ascl1, but negative for both Notch receptors and Hes1, while lung non-neuroendocrine cells are negative for Ascl1, but positive for Notch receptors and Hes1 (Figure 2A). This mutually exclusive expression pattern is true in lung cancers; as also confirmed by western blotting analyses, which revealed that SCLC cell lines are positive for ASCL1 and/or INSM1, but negative for Notch1, and NSCLC cell lines are negative for ASCL1 and/or INSM1, but positive for Notch1.
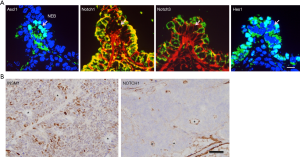
The combined type SCLC has both SCLC and NSCL compartments (18). Immunohistochemically, the SCLC compartment is positive for INSM1, but negative for Notch1, and the NSCLC compartment is negative for INSM1, but positive for Notch1 (Figure 2B), which suggests that Notch signaling pathway is important in determination of the subtypes of SCLC. Some molecular mechanisms of down-regulation of ASCL1 by Notch signaling have been proposed. The human ASCL1 promoter region has broad transcriptional enhancer and tissue-restricted transcriptional repressor motifs (31), and the repressor motif, an N-box sequence, is sensitive to Notch signaling activity via Hes1 binding (32). Moreover, Notch signaling can induce degradation of ASCL1 through proteasome activation (15). A combination of inactivation of Notch signaling, with expression of ASCL1, direct lung epithelial cells to a neuroendocrine phenotype (30), and this molecular relationship seems to be essential in the origin of SCLC (6).
Molecular mechanisms of small cell carcinogenesis
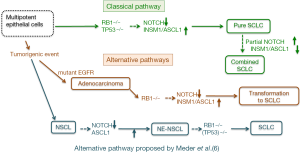
Frequent mutations in both TP53 and RB1 have been identified in SCLC cells (3-5), and thus it seems reasonable that genetic events with the bi-allelic TP53 and RB1 mutations must determine the process of small cell carcinogenesis. Actually, the experimental study by Meuwissen et al. (33) showed that mice carrying conditional alleles for both Trp53 and Rb1 developed small cell carcinoma in the lung, which supports the fact that inactivation of both Tp53 and RB1 is a prerequisite event for the pathogenesis of SCLC. Following this study, several mouse models which developed SCLC, have abnormalities in both p53 and Rb1 genes, and detailed analyses of the pathobiology of these SCLC in these models is useful to understand human SCLC (34). According to Meder et al. (6), SCLC has two oncogenic pathways: primary SCLC, which comes from neuroendocrine precursor cells, with bi-allelic TP53 and RB1 mutations, and secondary SCLC, which comes from Notch-defective NSCLC, which already has TP53 mutations and acquire additional RB inactivation.
Regarding to the origin of primary SCLC, they consider that neuroendocrine precursor cells -which are characterized by inactivation of Notch signaling and ASCL1 expression- are the origin of primary SCLC. This hypothesis could be accepted, as in mouse developing lungs, ASCL1 expressing cells could be a progenitor for various epithelial and mesenchymal cells (11), and could have migrating activity (12). However, using cell lineage-restricted Adeno-Cre virus, Sutherland et al. (35) showed clearly that loss of Tp53 and Rb1 could efficiently transform neuroendocrine, Clara and type 2 alveolar cells into SCLC cells (35). This study suggests that these epithelial cell lineages could be the origin of SCLC, and that inactivation of Notch signaling and ASCL1 expression are not always necessary to initiate SCLC development. Regarding to the origin of secondary SCLC, transformation from NSCLC to SCLC has been noticed, as a result of acquisition of resistance mechanisms against EGFR tyrosine kinase inhibitors (36). Niederst et al. (37) reported that such transformation from adenocarcinoma to SCLC always accompany the loss of RB1 gene, yet RB1 gene knockdown did not induce neuroendocrine differentiation in the EGFR mutant adenocarcinoma cell line. The combined SCLC cases presented by Meder et al. (6), suggest that NSCLC harboring Notch abnormalities, could become SCLC, with the addition of RB1 gene mutations, although abnormalities were reported in Notch2 but not in Notch1, and that inactivation of Notch2 is not a strong inducer of neuroendocrine differentiation (16). Considering that adenocarcinoma with mutant EGFR could transform to SCLC with the addition of RB1 gene abnormalities, the second and alternative pathway could be important in carcinogenesis of combined type SCLC. However, it is necessary to emphasize that combined type SCLC could originate from pure SCLC, and in this context, NSCL component should have active Notch signaling pathway and decreased ASCL1/INSM1 expression, contrary to Notch signaling inactivation and ASCL1/INSM1 expression seen in SCLC (18). Meder et al. (6) seems to emphasize -in their article- that a combination of inactivation of Notch signaling, with Ascl1 expression, precedes RB1 gene mutation, in small cell carcinogenesis, in both the classical and the alternative pathways. This issue may be similar to the question of which came first; the chicken or the egg. As SCLC could arise from different cell lineages, other than neuroendocrine precursor cells (35), the premise of inactivation of Notch signaling and ASCL1 expression is not always necessary to be considered. Prerequisite of genetic alterations in Tp53 and RB1 should be also crucial in the classical pathway for small cell carcinogenesis, and could be important in the alternative pathway in SCLC transformation from adenocarcinoma with mutant EGFR (Figure 3). It is an attractive research filed to clarify molecular network linking the inactivation of Notch signaling pathway, with ASCL1/INSM1 expression and RB1 gene abnormalities.
Acknowledgments
Funding: This study was supported in part by a Grant-in-Aid for Scientific Research (C; No. 25460439) from Ministry of Education, Culture, Sports, Science and Technology of Japan and in part by a grant from the Smoking Research Foundation.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shao-Hua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.03.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brambilla E, Gazdar A, Powell CA, et al. Neuroendocrine tumours small cell carcinoma. In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO classification tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: IARC Press; 2015:63-8.
- Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Meder L, König K, Ozretić L, et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927-38. [Crossref] [PubMed]
- Ito T. Differentiation and proliferation of pulmonary neuroendocrine cells. Prog Histochem Cytochem 1999;34:247-322. [Crossref] [PubMed]
- Cutz E, Yeger H, Pan J, et al. Pulmonary neuroendocrine cell system in health and disease. Curr Respir Med Rev 2008;4:174-86. [Crossref]
- Borges M, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997;386:852-5. [Crossref] [PubMed]
- Ito T, Udaka N, Yazawa T, et al. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000;127:3913-21. [PubMed]
- Li Y, Linnoila RI. Multidirectional differentiation of Achaete-Scute homologue-1-defined progenitors in lung development and injury repair. Am J Respir Cell Mol Biol 2012;47:768-75. [Crossref] [PubMed]
- Kuo CS, Krasnow MA. Formation of a Neurosensory Organ by Epithelial Cell Slithering. Cell 2015;163:394-405. [Crossref] [PubMed]
- Osada H, Tomida S, Yatabe Y, et al. Roles of achaete-scute homologue 1 in DKK1 and E-cadherin repression and neuroendocrine differentiation in lung cancer. Cancer Res 2008;68:1647-55. [Crossref] [PubMed]
- Osada H, Tatematsu Y, Yatabe Y, et al. ASH1 gene is a specific therapeutic target for lung cancers with neuroendocrine features. Cancer Res 2005;65:10680-5. [Crossref] [PubMed]
- Sriuranpong V, Borges MW, Strock CL, et al. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol 2002;22:3129-39. [Crossref] [PubMed]
- Morimoto M, Nishinakamura R, Saga Y, et al. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012;139:4365-73. [Crossref] [PubMed]
- Wael H, Yoshida R, Kudoh S, et al. Notch1 signaling controls cell proliferation, apoptosis and differentiation in lung carcinoma. Lung Cancer 2014;85:131-40. [Crossref] [PubMed]
- Fujino K, Motooka Y, Hassan WA, et al. Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am J Pathol 2015;185:3164-77. [Crossref] [PubMed]
- Ballas N, Grunseich C, Lu DD, et al. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 2005;121:645-57. [Crossref] [PubMed]
- Kreisler A, Strissel PL, Strick R, et al. Regulation of the NRSF/REST gene by methylation and CREB affects the cellular phenotype of small-cell lung cancer. Oncogene 2010;29:5828-38. [Crossref] [PubMed]
- Ishii J, Sato H, Sakaeda M, et al. POU domain transcription factor BRN2 is crucial for expression of ASCL1, ND1 and neuroendocrine marker molecules and cell growth in small cell lung cancer. Pathol Int 2013;63:158-68. [Crossref] [PubMed]
- Jia S, Wildner H, Birchmeier C. Insm1 controls the differentiation of pulmonary neuroendocrine cells by repressing Hes1. Dev Biol 2015;408:90-8. [Crossref] [PubMed]
- Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development 2004;131:4299-310. [Crossref] [PubMed]
- Parisi T, Yuan TL, Faust AM, et al. Selective requirements for E2f3 in the development and tumorigenicity of Rb-deficient chimeric tissues. Mol Cell Biol 2007;27:2283-93. [Crossref] [PubMed]
- Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene 2008;27:5124-31. [Crossref] [PubMed]
- Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev 2007;17:52-9. [Crossref] [PubMed]
- Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001;61:3200-5. [PubMed]
- Hassan WA, Yoshida R, Kudoh S, et al. Notch1 controls cell invasion and metastasis in small cell lung carcinoma cell lines. Lung Cancer 2014;86:304-10. [Crossref] [PubMed]
- Hassan WA, Yoshida R, Kudoh S, et al. Notch1 controls cell chemoresistance in small cell lung carcinoma cells. Thorac Cancer 2016;7:123-8. [Crossref] [PubMed]
- Ball DW. Achaete-scute homolog-1 and Notch in lung neuroendocrine development and cancer. Cancer Lett 2004;204:159-69. [Crossref] [PubMed]
- Chen H, Biel MA, Borges MW, et al. Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions. Cell Growth Differ 1997;8:677-86. [PubMed]
- Chen H, Thiagalingam A, Chopra H, et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A 1997;94:5355-60. [Crossref] [PubMed]
- Meuwissen R, Linn SC, Linnoila RI, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 2003;4:181-9. [Crossref] [PubMed]
- Gazdar AF, Savage TK, Johnson JE, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol 2015;10:553-64. [Crossref] [PubMed]
- Sutherland KD, Proost N, Brouns I, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754-64. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [Crossref] [PubMed]
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [Crossref] [PubMed]


