
Hypoxic cell sensitization in chemoradiation for cervical cancer
The integration of cisplatin into radiation regimens was first developed in locally advanced cervical cancer, a tumour where there is the added advantage that high dose intracavity radiation can be given as well as external beam therapy (1), and the concept was later extended to head and neck cancers. However, progress since then has been slow with the addition of other radiosensitising drugs like gemcitabine providing only a modest survival advantage (2). Myelosuppression and gastrointestinal toxicity also became dose limiting with the doublet. DiSilvestro and colleagues reported a phase III trial of cisplatin based chemoradiation with or without the hypoxic cell sensitizer tirapazamine in 402 predominantly Caucasian and non-Hispanic patients with stages IB, IIa, IIIB and IVa cervical cancer in 2014 (3). The GOG 219 study took 3.5 years to accrue and was closed in 2009 prematurely on account of lack of study drug.
The study was well conducted with an adequate dose and schedule of radiation and acute toxicity was within acceptable limits, although vomiting, diarrhea and neuropathy were greater in the tirapazamine arm. Node sampling was recorded as performed in >80% of cases, but the results are not reported. Similarly results have not been presented for parallel translational studies. The study was negative with no PFS or OS benefit for the addition of tirapazamine.
Tirapazamine can be described as a second generation sensitiser based on a benzotriazine backbone which is bioreductively activated in hypoxic cells (4). In cell lines it had previously been shown to be up to 450× more cytotoxic in hypoxic compared to well-oxygenated cells, producing both single strand and double strand breaks. Earlier studies with 2-nitroimidazoles in both cervix and head and neck cancer were often underpowered and no overall effect on outcome was shown. Tirapazamine also has an inhibitory effect on DNA repair (5) and in vitro and in vivo synergy with cisplatin had been demonstrated. This last finding may be of significance because GOG 219, in common with many other randomized studies of cisplatin based chemoradiation, had shown a lower distant metastatic rate. The individual patient meta-analysis carried out in 2008 showed an absolute risk reduction of death of 6% and a 7% reduction in distant metastases by the addition of cisplatin to radiation (6).
The background to the study was preclinical work suggesting synergy between tirapazamine and cisplatin confirmed by phase II studies of this combination not only in advanced cervical cancer with a response rate of the order of 30% (7,8), but also in other tumour types. There was one phase I study of 15 patients in chemoradiation in which the dose of cisplatin was halved and the tirapazamine was administered on days 8, 10, 12, 22, 24 and 26 (9) which led to the phase III study. Subsequently two randomized head and neck cancer studies recruiting 923 patients to assess the addition of tirapazamine to cisplatin based chemoradiation showed no survival gain but increased myelosuppression in the experimental arm (10,11).
The 2008 meta-analysis furthermore identified two studies employing additional chemotherapy after completion of chemoradiation in cervical cancer, and suggested this may be associated with a gain in survival. Subsequent analysis of the subgroups of the cisplatin/gemcitabine study showed that there was a survival gain associated with the continuation of chemotherapy after the chemoradiation (2). There are two confirmatory studies in progress addressing the issue of additional adjuvant chemotherapy (ANZGOG 0972/GOG 0274 and RTOG 0724).
The above studies demonstrate that additional concomitant chemotherapy to cisplatin adds to toxicity without significant benefit, and raise the question whether the activity of tirapazamine is related to its hypoxic selectivity or to sensitization of cisplatin. A further concern is the extent to which chronic or transient hypoxia exists in tumours of different size and vascularity, as well as the known heterogeneity in the oxygen tension measured in different parts of the same tumour (12). The message emerging over the last 10 years is that unless clinically relevant biomarkers are available, translation to clinical studies will risk rejection of promising compounds and premature progression to large phase III trials will not be cost-effective.
There have also been recent advances in our understanding of the genomics of cervical cancer and of the adaptive responses to hypoxia, which are largely mediated by HIF1α leading to transcriptional activation of genes which reduce cellular oxygen demand. There is also epigenetic repression of DICER leading to an epithelial to mesenchymal transition and acquisition of stem cell and metastatic phenotypes (13). Whole exome sequencing of 115 cervical carcinomas was reported in 2014 and besides showing differences between squamous and adenocarcinomas, suggested that there was increased expression of adjacent genes as a result of HPV integration and these included the growth factor ERB2 (14). The most frequently mutated gene set was also shown to involve immune response genes.
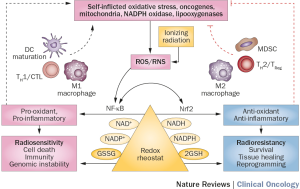
Ionizing radiation itself may disrupt the immune system as a result of the acute inflammation produced, and reactive oxygen species (ROS) may promote antigen presentation leading to activation of a range of cytokines and cellular responses depending on the redox status of the microenvironment (Figure 1). Several preclinical and clinical observations have led to interest in combining immune checkpoint inhibitors with radiation (16). Once the initial studies are complete, combination of radiosensitisers with immunotherapy as an adjunct to chemoradiation will be worth exploring, either concomitantly or sequentially.
There has also been renewed interest in exploiting DNA repair pathways largely as a result of the development of the PARP inhibitors as cytotoxic agents in BRCA deficient tumours. These agents produce single strand breaks and in combination with the HR defect associated with BRCA deficiency and likely other DNA repair deficiencies, can induce a state of synthetic lethality (17). Antimetabolites used as radiosensitisers including 5-FU and gemcitabine target homologous repair (HR) while cisplatin predominantly targets non-homologous end joining (18). Evidence of radiosensitisation by the PARP inhibitors has led to the initiation of a number of clinical trials in several tumour types targeting DNA repair molecules including ATM, Chk1/2 and WEE1 (19).
In summary, there are a number of therapeutic possibilities for enhancing radiation response, including hypoxic sensitization, DNA repair, growth factor inhibition and immunomodulation (20). The interactions between these processes are beginning to become clear. As these are developed, researchers will have to deal with the difficulties of drug and radiation interactions, tumour heterogeneity and stem cell plasticity. However, cervical cancer remains one of the best systems to evaluate these effects.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.03.05). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358:781-6. [Crossref] [PubMed]
- Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011;29:1678-85. [Crossref] [PubMed]
- DiSilvestro PA, Ali S, Craighead PS, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. J Clin Oncol 2014;32:458-64. [Crossref] [PubMed]
- Brown JM, Lemmon MJ. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res 1990;50:7745-9. [PubMed]
- Dorie MJ, Brown JM. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Res 1993;53:4633-6. [PubMed]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802-12. [Crossref] [PubMed]
- Maluf FC, Leiser AL, Aghajanian C, et al. Phase II study of tirapazamine plus cisplatin in patients with advanced or recurrent cervical cancer. Int J Gynecol Cancer 2006;16:1165-71. [Crossref] [PubMed]
- Smith HO, Jiang CS, Weiss GR, et al. Tirapazamine plus cisplatin in advanced or recurrent carcinoma of the uterine cervix: a Southwest Oncology Group study. Int J Gynecol Cancer 2006;16:298-305. [Crossref] [PubMed]
- Rischin D, Peters L, Hicks R, et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol 2001;19:535-42. [PubMed]
- Le QT, Taira A, Budenz S, et al. Mature results from a randomized Phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable Stage IV head and neck squamous cell carcinomas. Cancer 2006;106:1940-9. [Crossref] [PubMed]
- Rischin D, Peters LJ, O'Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010;28:2989-95. [Crossref] [PubMed]
- Hill RP, Bristow RG, Fyles A, et al. Hypoxia and Predicting Radiation Response. Semin Radiat Oncol 2015;25:260-72. [Crossref] [PubMed]
- van den Beucken T, Koch E, Chu K, et al. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nat Commun 2014;5:5203. [Crossref] [PubMed]
- Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014;506:371-5. [Crossref] [PubMed]
- Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol 2015;12:527-40. [Crossref] [PubMed]
- Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013;85:293-5. [Crossref] [PubMed]
- Noël G, Godon C, Fernet M, et al. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol Cancer Ther 2006;5:564-74. [Crossref] [PubMed]
- Sears CR, Turchi JJ. Complex cisplatin-double strand break (DSB) lesions directly impair cellular non-homologous end-joining (NHEJ) independent of downstream damage response (DDR) pathways. J Biol Chem 2012;287:24263-72. [Crossref] [PubMed]
- Morgan MA, Parsels LA, Maybaum J, et al. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov 2014;4:280-91. [Crossref] [PubMed]
- Sagae S, Monk BJ, Pujade-Lauraine E, et al. Advances and Concepts in Cervical Cancer Trials: A Road Map for the Future. Int J Gynecol Cancer 2016;26:199-207. [Crossref] [PubMed]


