
Proton therapy for head and neck cancer: current applications and future directions
Radiation therapy for head and neck cancer: indications and challenges
External beam radiation therapy (EBRT) is a well-established therapeutic modality in the treatment of head and neck cancer, with more than 80% of patients diagnosed with head and neck cancer receiving EBRT as a portion of their therapy. For early-stage cancers, it is often used as the primary treatment, obtaining high local control rates with limited fields (1,2). For locally advanced cancers, it is the standard treatment for cancers not amenable to surgical resection, such as nasopharynx cancer (3), and as an organ-preserving alternative to surgery for cancers such as larynx cancer (4,5). In the post-operative setting for locally-advanced disease with high-risk pathologic features, adjuvant EBRT can improve locoregional control and survival when given alone (6), or in conjunction with chemotherapy (7-9).
EBRT to the head and neck is associated with acute and late toxicity. The need to irradiate areas of disease involvement, which are in close proximity to normal tissues, results in significant radiation exposure to these tissues, with toxicity observed early in the course of treatment. Dose to the oral mucosa results in mucositis, a common side effect which can cause severe pain, difficulty swallowing, and malnutrition due to the inability to eat. Other common acute effects include xerostomia and dysgeusia. These side effects can lead to hospitalization and treatment interruptions (10), which may ultimately adversely affect disease outcomes (11).
Late effects secondary to head and neck radiation are also of concern. Dose to the cochlea can cause hearing loss, particularly in those who have also received platinum-based chemotherapy. Radiation exposure to the salivary glands causes chronic xerostomia, which can affect eating, communication, pain, and emotion (12), as well as increase the risk of developing dental caries. Patients receiving high doses of radiation to the mandible are at risk for mandibular osteoradionecrosis (13), especially if they require post-radiation dental extractions. Exposure of swallowing structures to radiation can lead to long-term dysphagia (14), aspiration, and chronic reliance on nutritional supplementation, such as via gastrostomy tube. Side effects of radiation to the neck include lymphedema, fibrosis, and hypothyroidism (15). Neck radiation can also potentially cause accelerated carotid atherosclerosis, as evidenced by an increased risk of ischemic stroke after RT in younger (16) and older patients (17). Due to the changing epidemiology of head and neck cancer, with an increasing proportion of younger patients developing human-papilloma virus (HPV) positive oropharynx cancer who are treated and cured at high rates (18), minimizing long-term radiation-related morbidity will become increasingly important.
Advances in photon-based external beam radiation therapy: 3-D conformal radiotherapy (3-D CRT) and intensity-modulated radiation therapy (IMRT)
Technical advancements in photon-based radiotherapy, such as with 3-D CRT and IMRT, allow for a more conformal deposition of the high-dose region and therefore, an improved therapeutic ratio. Three-dimensional conformal planning utilizes multiple radiation beams shaped by a static multileaf collimator in an effort to better conform radiation dose to the targets of interest. IMRT further improves this process, through the use of a dynamic multileaf collimator that can modulate both the shape and intensity of individual beams to create an optimal dose distribution to treat disease and further spare normal tissues (Figure 1). The addition of daily image guidance (IGRT) has led to a decrease in the planning target volume (PTV) for radiation, which has the potential of decreasing normal tissue exposure to high-dose radiation without compromising locoregional control (19).
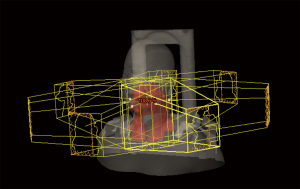
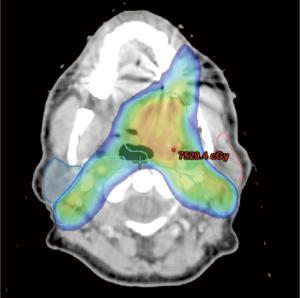
Although direct comparisons of IMRT to conventional radiation are limited, the literature supports its use given the promising results obtained with respect to disease control, toxicity, and quality of life. The University of California-San Francisco has reported their experience of treating nasopharynx cancer with IMRT (20). A total of 35 patients were treated, and at a median follow-up of 21.8 months, locoregional control was 100%. An update of their experience, which included 67 patients with a median follow-up of 31 months, continued to show an excellent 4-year locoregional control rate of 98% (21). Compared to conventional radiation, IMRT is superior in its ability to reduce dose to critical normal organs. An example of this is sparing the parotid gland (Figure 2) to minimize risk of long-term xerostomia, which can impair quality of life (12). A matched case-control study comparing IMRT to standard radiotherapy for head and neck cancer found that xerostomia and quality of life improved over time (starting at 6 months post-treatment) after IMRT, but not after standard RT (22). A phase III multicenter trial (PARSPORT) randomized 94 patients to receive either IMRT or conventional RT and found that parotid-sparing IMRT significantly reduced the incidence of long-term xerostomia, and improved quality of life (23).
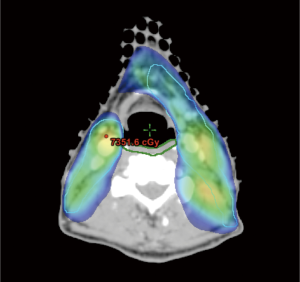
IMRT has also been used in the context of comprehensive nodal radiation of the neck to spare swallowing structures and minimize risk of long-term dysphagia (Figure 3). A prospective, clinical study of 73 patients with stage III or IV oropharynx cancer treated with concurrent chemotherapy and IMRT at the University of Michigan found that efforts to spare the pharyngeal constrictors with IMRT could be done safely (3-year locoregional control 96%), and effectively (only 1 patient was feeding tube dependent 12 months after completion of IMRT) (24). They reported that long-term measures of swallowing were only slightly worse than pre-therapy baseline levels, and found a correlation between the mean doses delivered to swallowing organs and long-term dysphagia (25). However, even with IMRT, toxicity remains a pertinent issue, with rates of long-term gastrostomy tube dependence as high as 20% (26), and impaired quality of life secondary to chronic xerostomia and dysphagia (27). Efforts to explore methods to decrease dose to normal tissues, such as with proton therapy, are therefore warranted.
Proton therapy: potential advantages
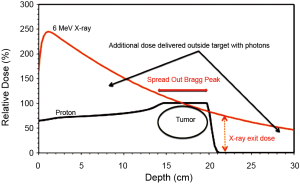
Unlike photon radiation, proton therapy offers the added advantages of less dose delivered to tissues proximal to the tumor and rapid dose fall off at the distal edge of the tumor (Bragg-Peak effect, Figure 4). This allows for potential gains with respect to normal organ sparing and provides opportunities for potential dose escalation. Applied in the treatment of head and neck cancer, proton therapy could be utilized in the following ways:
(I) Dose escalation for cancers where locoregional control is currently limited by an inability to adequately deliver therapeutic doses without excessive risk of toxicity.
(II) Minimizing exposure of normal tissues and decreasing toxicity in patients for whom long-term control is obtained with currently-prescribed doses, but at the cost of potential significant toxicity.
Multiple comparative planning studies have shown that the dose distribution attainable with proton therapy appear superior to those possible with photon radiation. Two separate studies from the Paul Scherrer Institute, each derived from the CT scans of 5 patients treated for head and neck cancer, explored the potential benefits of proton radiotherapy compared to conventional treatment. The first study, in which 3-D conformal radiation was compared to IMRT and proton therapy (passively scattered and spot scanned), demonstrated that proton therapy provided the best dose homogeneity with respect to PTV coverage, as well as spinal cord and parotid gland sparing (28). The second study, a comparison of IMRT versus intensity-modulated proton therapy (IMPT) showed that critical organs were optimally spared with IMPT, with lower estimated secondary cancer risks as a result of lower integral dose received by normal tissue (29). Reduced second-malignancy risk also appears to be an advantage for non-IMPT, double-scatter proton therapy (30), even despite concerns about secondary neutrons from protons causing second malignancies. This risk should be significantly lower with the implementation of IMPT, given the reduced secondary neutron scatter associated with this technique.
For treatment of sinonasal tumors (for which adequate dose delivery is often limited due to the proximity of normal organs), proton-based planning was superior to conventional, conformal, as well as IMRT for normal organ sparing (31), while IMPT was superior to IMRT in sparing normal organs at both low- and high-dose levels (32). To study the potential gains with respect to long-term dysphagia, van der Laan et al. (33) conducted a comparative study in which IMPT plans were generated for 25 patients who were treated with IMRT to the bilateral neck for oropharynx or hypopharynx cancer. In an adaptive planning study, initial and re-simulation CT images from 10 patients with head and neck cancer were used to compare differences in doses to normal structures with non-adaptive and adaptive IMRT and IMPT replanning (34). Adaptive IMPT significantly reduced doses to multiple critical structures when compared against non-adaptive IMPT, and reduced doses to all critical structures when compared to non-adaptive and adaptive photon planning.
While planning studies clearly show the dosimetric advantages of proton therapy over photon radiation, clinical implementation and correlation to outcomes have been largely limited to small, single-institution series. Early results of local control appear promising, especially in anatomic sites in which organs at risk currently limit delivery of adequate photon doses. The Massachusetts General Hospital reported a 2-year locoregional control rate of 86% in their series of 20 patients with locally advanced sphenoid sinus malignancies treated with proton beam to a median dose of 76 Gy (35). Treatment appears well-tolerated, as evidenced by the published acute and late-toxicity rates. Tokuuye et al. reported toxicity results on 33 patients who received definitive proton therapy to a median dose of 76 Gy, at 2.8 Gy per fraction, with one (3%) and six (18%) patients experiencing > grade 3 acute and late toxicity, respectively (36). The Heidelberg ion therapy center published one of the largest series, in which 118 patients with skull base tumors were treated with proton and carbon ion radiotherapy (37). Few side effects were observed, which were mainly grade 1. The administration of large doses per fraction with protons also appears safe. In a pilot study of 14 patients with mucosal melanoma of the head and neck treated with proton therapy three times per week for 15 fractions to a total dose of 60 Gy, all patients were able to receive the full dose of therapy (38). Initial local control was 85.7%, and at a median follow-up of 3 years, there were no treatment-related deaths. Twenty-one percent of patients experienced grade 3 mucositis, 2 patients had unilateral decrease in visual acuity.
Although these data are encouraging, there are several factors which limit the ability to draw definitive conclusions regarding proton therapy. First, proton therapy is associated with uncertainties in dose delivery typically related to uncertainty regarding the precise location of the distal edge of the Bragg peak (39-42). Second, many of the dosimetric advantages of proton radiotherapy seen in planning studies were achieved with pencil-beam scanning and IMPT, a modality which still requires further technical development and ideally, means for in vivo range verification prior to clinical implementation. Third, worldwide, there are still relatively few proton therapy centers, which has limited the ability to treat and analyze a large number of patients and determine the most appropriate indications for proton therapy. Additional comparative effectiveness research is needed to best understand the benefit of proton therapy for specific patient populations and clinical conditions (43).
Current indications and future applications: the University of Pennsylvania experience
At our institution, there are several indications for delivering proton therapy for head and neck cancer. One indication is for treating patients with salivary gland cancers. Previously, these patients were treated with IMRT, but are now currently treated with double scattering or uniform scanning proton therapy, as shown in Figure 5. When compared to IMRT, proton therapy can decrease dose to adjacent normal organs such as the brainstem, cochlea, temporal lobe, and the contralateral salivary glands. Other dosimetric advantages include limiting the area of low dose radiation delivered to normal tissues. These dosimetric gains could potentially translate to improved long-term results such as decreasing rates of chronic xerostomia and radiation-induced secondary malignancies. The potential decrease in radiation-induced malignancy with proton therapy is of particular importance, given the increasing incidence of oropharynx cancer (44), which is typically diagnosed in younger patients, and for whom long-term disease control is likely (18).
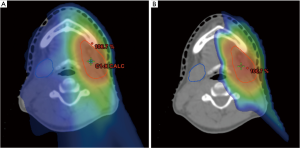
Pencil beam scanning is being used for the treatment of base of skull malignancies. Treatment of tumors at this particular site with conventional radiation has traditionally been limited by an inability to deliver adequate doses of radiation without exceeding constraints on critical structures in the brain and optic apparatus. Unlike double scattering or uniform scanning proton therapy, pencil beam scanning allows for enhanced conformal dose around critical structures through modulation of dose in depth, while retaining the rapid dose fall-off from the Bragg-Peak effect (Figure 6). For both of these indications, it is critical to enroll patients on clinical trials or registries to collect outcome data, thereby assessing the effectiveness and role of proton therapy.
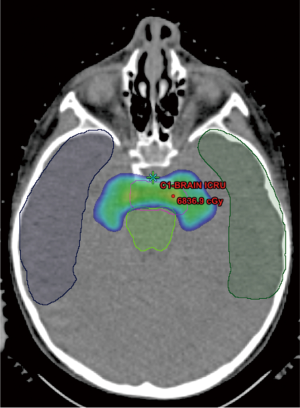
Another indication is for reirradiation for recurrent head and neck cancer. Patients who require head and neck reirradiation generally have poor outcomes, with median survival typically less than 12 months, and reirradiation limited by treatment-related morbidity (45-48). Proton therapy, by potentially allowing for high-dose reirradiation while decreasing normal tissue exposure, may lead to improved outcomes. Lin et al. reported results on 16 patients reirradiated with protons for recurrent nasopharyngeal carcinoma (49), for which 2-year local control and overall survival were approximately 50%. Priority was given to minimizing toxicity (no patients experienced CNS toxicity) over tumor coverage. The 2-year survival was significantly higher in those with “optimal” dose-volume histogram coverage versus those with “suboptimal” coverage (83% and 17%, respectively, P=0.006). Patients who require head and neck reirradiation with proton therapy at the University of Pennsylvania are currently enrolled on clinical study, with the hopes that improving coverage of affected areas while minimizing normal tissue toxicity can improve clinical outcomes in a population that otherwise has limited options.
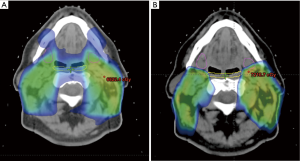
Current efforts include the development of pencil beam scanning proton therapy for treatment of the comprehensive, bilateral neck, which is required in the majority of patients with locally advanced mucosal squamous cell carcinoma of the head and neck. In order to take full dosimetric advantage of proton therapy, treatment requires a small beam spot size, which can be difficult to achieve when treating a superficial target, such as the neck. Presently at our institution, the minimum deliverable energy for pencil beam scanning is 100 MeV, requiring the use of a range shifter for treatment of targets that extend within 7.5 cm water-equivalent depth of the skin surface. There is a large air gap, typically greater than 30 cm, between the range shifter and patient surface through which spots scattered in the range shifter increase in size before reaching the target. The incorporation of a tissue-equivalent bolus that conforms and can be placed over the skin of the neck decreases the spot size of the beam at the depth of the neck lymphatics by eliminating the large air gap between the bolus and the patient. Dosimetric plans utilizing such a system show quite promising potential gains compared to IMRT, with possible further sparing of the swallowing structures (Figure 7), as well as the ability to protect structures not currently spared via IMRT, such as the submandibular glands (Figure 7). We plan to begin treatment of the comprehensive, bilateral neck within the next year by using such an approach. Other technical challenges specific to proton therapy include uncertainties in estimating proton stopping power from the planning CT image especially in cases with substantial CT image artifacts and sensitivity to anatomical changes such as patient setup or weight loss that may impact the dose distribution. Further research to quantify and minimize the impact of image artifacts is necessary to ensure robust proton therapy. Adaptive therapy and replanning during the course of therapy may be a clinical necessity in proton therapy given the dosimetric sensitivity to anatomical changes.
While from a dosimetric perspective, proton therapy appears superior to IMRT, it is still unclear whether these physical advantages translate to improved clinical outcomes. Therefore, the importance of enrolling patients who are to receive proton therapy on clinical studies cannot be overstated. These studies should have carefully described clinical endpoints, such as disease control, toxicity, and quality of life, and patients receiving proton therapy should ideally be compared to a control cohort receiving IMRT. At our institution, the goal is for all patients receiving proton therapy to be enrolled on a clinical study and/or registry. In patients receiving CNS or base of skull proton therapy, neuropsychiatric testing is performed routinely before, during and after treatment to assess the neurocognitive changes secondary to RT. In our pending implementation of bilateral neck proton therapy, we plan to assess patients with objective, functional swallow testing as well as with general, head and neck, and xerostomia-specific quality of life inventories prior, during, and after treatment. Results will then be compared to a matched cohort of patients receiving IMRT, in order to correlate dosimetric with clinical advantages.
Conclusions
Proton therapy is a promising and emerging modality of radiation therapy for patients with head and neck cancers. The physical advantages inherent to protons, with rapid dose fall off, can yield improvements in the ability to escalate radiation dose, or to better spare organs at risk. Although emerging clinical data are promising, new techniques, such as pencil beam scanning and IMPT need to be developed further in order to overcome current limitations, and to potentially expand the indications under which proton therapy should be considered. Patients should ideally be treated on clinical study and compared, when possible, to a similar cohort of patients treated with IMRT.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Huan Giap and Eric Y Chuang) for the series “Particle Beam Therapy II” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.12.01). The series “Particle Beam Therapy II” was commissioned by the editorial office without any funding or sponsorship. None of the authors report any relevant disclosures or conflicts of interest as it pertains to this manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys 2006;64:77-82. [PubMed]
- O’Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys 2001;51:332-43. [PubMed]
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [PubMed]
- . Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 1991;324:1685-90. [PubMed]
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8. [PubMed]
- Lundahl RE, Foote RL, Bonner JA, et al. Combined neck dissection and postoperative radiation therapy in the management of the high-risk neck: a matched-pair analysis. Int J Radiat Oncol Biol Phys 1998;40:529-34. [PubMed]
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945-52. [PubMed]
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937-44. [PubMed]
- Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005;27:843-50. [PubMed]
- Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003;66:253-62. [PubMed]
- Cox JD, Pajak TF, Marcial VA, et al. Interruptions adversely affect local control and survival with hyperfractionated radiation therapy of carcinomas of the upper respiratory and digestive tracts. New evidence for accelerated proliferation from Radiation Therapy Oncology Group Protocol 8313. Cancer 1992;69:2744-8. [PubMed]
- Lin A, Kim HM, Terrell JE, et al. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys 2003;57:61-70. [PubMed]
- Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and Radiation Dose to the Mandible in Patients With Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys 2013;85:415-20. [PubMed]
- Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002;53:23-8. [PubMed]
- Smith GL, Smith BD, Garden AS, et al. Hypothyroidism in older patients with head and neck cancer after treatment with radiation: a population-based study. Head Neck 2009;31:1031-8. [PubMed]
- Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol 2002;20:282-8. [PubMed]
- Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol 2008;26:5119-25. [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [PubMed]
- Chen AM, Farwell DG, Luu Q, et al. Evaluation of the planning target volume in the treatment of head and neck cancer with intensity-modulated radiotherapy: what is the appropriate expansion margin in the setting of daily image guidance? Int J Radiat Oncol Biol Phys 2011;81:943-9. [PubMed]
- Sultanem K, Shu HK, Xia P, et al. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: the University of California-San Francisco experience. Int J Radiat Oncol Biol Phys 2000;48:711-22. [PubMed]
- Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002;53:12-22. [PubMed]
- Jabbari S, Kim HM, Feng M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys 2005;63:725-31. [PubMed]
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127-36. [PubMed]
- Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol 2010;28:2732-8. [PubMed]
- Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys 2011;81:e93-9. [PubMed]
- Ingle CJ, Yip K, Caskie V, et al. Intensity modulated radiotherapy (IMRT) in the management of locally advanced oropharyngeal squamous cell carcinomata (SCC): disease control and functional outcome using the therapy outcome measure (TOM) score--report from a single U.K. institution. Head Neck Oncol 2010;2:28. [PubMed]
- Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008;26:3770-6. [PubMed]
- Cozzi L, Fogliata A, Lomax A, et al. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol 2001;61:287-97. [PubMed]
- Steneker M, Lomax A, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol 2006;80:263-7. [PubMed]
- Yoon M, Ahn SH, Kim J, et al. Radiation-induced cancers from modern radiotherapy techniques: intensity-modulated radiotherapy versus proton therapy. Int J Radiat Oncol Biol Phys 2010;77:1477-85. [PubMed]
- Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2004;58:147-54. [PubMed]
- Lomax AJ, Goitein M, Adams J. Intensity modulation in radiotherapy: photons versus protons in the paranasal sinus. Radiother Oncol 2003;66:11-8. [PubMed]
- van der Laan HP, van de Water TA, van Herpt HE, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol 2013;52:561-9. [PubMed]
- Simone CB 2nd, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol 2011;101:376-82. [PubMed]
- Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies: treatment outcome and prognostic factors. Head Neck 2009;31:1297-308. [PubMed]
- Tokuuye K, Akine Y, Kagei K, et al. Proton therapy for head and neck malignancies at Tsukuba. Strahlenther Onkol 2004;180:96-101. [PubMed]
- Rieken S, Habermehl D, Nikoghosyan A, et al. Assessment of early toxicity and response in patients treated with proton and carbon ion therapy at the Heidelberg ion therapy center using the raster scanning technique. Int J Radiat Oncol Biol Phys 2011;81:e793-801. [PubMed]
- Zenda S, Kawashima M, Nishio T, et al. Proton beam therapy as a nonsurgical approach to mucosal melanoma of the head and neck: a pilot study. Int J Radiat Oncol Biol Phys 2011;81:135-9. [PubMed]
- Minohara S, Endo M, Kanai T, et al. Estimating uncertainties of the geometrical range of particle radiotherapy during respiration. Int J Radiat Oncol Biol Phys 2003;56:121-5. [PubMed]
- Engelsman M, Kooy HM. Target volume dose considerations in proton beam treatment planning for lung tumors. Med Phys 2005;32:3549-57. [PubMed]
- Lu HM. A potential method for in vivo range verification in proton therapy treatment. Phys Med Biol 2008;53:1413-24. [PubMed]
- Lu HM. A point dose method for in vivo range verification in proton therapy. Phys Med Biol 2008;53:N415-22.
- Bekelman JE, Shah A, Hahn SM. Implications of comparative effectiveness research for radiation oncology. Practical Radiation Oncology 2011;1:72-80.
- Marur S, D’Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010;11:781-9. [PubMed]
- De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol 1998;16:3556-62. [PubMed]
- Salama JK, Vokes EE, Chmura SJ, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2006;64:382-91. [PubMed]
- Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol 2007;25:4800-5. [PubMed]
- Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008;30:281-8. [PubMed]
- Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy--dose-volume histogram analysis. Radiology 1999;213:489-94. [PubMed]


