Effect of endotoxin exposure on lung cancer risk in cotton textile mills and agriculture: a meta-analysis
Introduction
Endotoxin, usually referred to as lipopolysaccharide, is a component of the outer membrane of Gram-negative bacteria (1). During the period of bacteria dissolution, endotoxin is released to develop its biological functions (1). Endotoxin is widespread in indoor and outdoor environments, especially in various workplaces generated amounts of organic dusts (2,3). High endotoxin concentrations are found in several certain occupational settings, such as cotton textile mills, agriculture work, saw industries and so on (2,3).
Inhaling endotoxin contaminated organic dusts can give rise to numerous acute and chronic respiratory diseases (3-5). In contrast, as early as 1973, Henderson et al. noticed that cotton-exposed workers showed a lower than expected mortality of lung cancer, but the exact substances and mechanisms inducing this phenomenon were not clear at that point (6). Subsequently, endotoxin was proven to have the antitumor function in animal models and clinical trials (7-9). To date, many epidemiology researches have reported an inverse association between endotoxin exposure and lung cancer risk (10-13). A meta-analysis incorporated 28 studies also showed a protective effect of endotoxin against lung cancer in cotton textile and agriculture workers (14). However, a promotional lung cancer risk with increasing exposure time or cumulative concentrations have been demonstrated in both an updated case-control study and a large pooled case-cohort study published recently (15,16).
Despite most epidemiological studies suggesting a decreased lung cancer risk exposure to endotoxin, few have measured concentrations quantitatively or adequately adjusted for smoking, which may weaken their conclusions. On account of these inconsistent results, we conducted a meta-analysis to investigate the relationship between endotoxin and lung cancer, focusing on two workplaces, agriculture and cotton textile industries, which highly contaminated by endotoxin (17).
Methods
Search strategies
Relevant articles were searched in PubMed, EMBASE, the Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese BioMedical Literature database (CBM), WanFang database and VIP database up to March, 2015 by two investigators (LYX and KW). Taking PubMed searches as an example, the strategy was as follows: {“lung cancer” OR “lung neoplasm” OR “pulmonary cancer” OR “pulmonary neoplasm” OR “bronchogenic carcinoma” OR “lung neoplasms [Mesh]”} AND (“endotoxin” OR “organic dust”) AND {“cotton” OR “cotton fiber [Mesh]” OR “textile” OR “textile industry [Mesh]” OR “farm” OR “farmers” OR “agriculture” OR “Agricultural Workers’ Diseases [Mesh]”}. Moreover, bibliographies of included studies and relevant reviews were scanned to identify additional studies. Meeting’s proceedings or abstracts were rejected. Languages were restricted to English and Chinese.
Study selection
Publications were considered to be eligible if they met the following inclusion criteria: (I) they took cotton textile or agricultural workers as participants with the purpose of exploring the effect of occupational endotoxin exposure on lung cancer risk; (II) they provided effect sizes [relative risk (RR) or odds ratio (OR) or hazard ratio (HR) or standardized mortality ratio (SMR) or standardized incidence ratio (SIR)] with the corresponding 95% CIs, or provided enough data to calculate them; (III) research types were limited to cohort, case-control and case-cohort studies. Studies that took other cancer or respiratory disease patients as controls, which may prone to selection bias, were excluded (14,18). When several articles from the same cohort were available, we only included the most recent paper or paper with the most applicable data.
Data collection and quality assessment
Two investigators (LYX and KW) extracted the data independently and discussed to reach a consensus. The following information was recorded: the first author’s name, year of publication, country or region, study design, cohort size, number of cases, follow-up period, adjustment for confoundings, exposure assessment and effect size with corresponding 95% CI. Since one cohort study didn’t directly provide an overall hazard ratio of lung cancer, we extracted original data from the paper for two-by-two tables and then estimated a crude relative risk (19). If a study didn’t report a 95% CI, we utilized the exact Possion confidence intervals or Byar’s approximation to calculated it (20). The methodological quality of included studies were assessed through the New-Castle Ottawa Scale (NOS) for observational studies (21). A study scored six or more stars was judged as high-quality (22).
Statistical analysis
As lung cancer incidence was rare in the population, we ignored the distinction among various risk estimates (RR, OR, HR, SMR, SIR) and expressed the pooled effect size as RRs (23,24). If a study separately reported effect sizes classified by gender or different exposure levels, we combined subgroup results into one overall risk by a random-effects model (22). Statistical heterogeneity across studies was quantified using I2 statistic (25). Heterogeneity was deemed to be statistical significant at P<0.10, in this instance, a random-effects model (DerSimonian and Laird method) should be applied to estimate summary effect size (26). Otherwise, a fixed-effects model (Mantel-Haenszel method) was utilized (27). To identify potential sources of heterogeneity, we performed the Galbraith radial plots (28) and subgroup analyses based on study design, sex, adjustment for smoking, region, outcome, follow-up period and farm type. Sensitivity analyses were conducted to assess the influence of each individual study on the summary relative risk. Potential publication bias was evaluated with Egger’s test (29) and Begg’s funnel plot (30).
All analyses were conducted by STATA, version 12.0 (Stata Corporation, College Station, TX, USA). P<0.05 denoted statistical significance.
Results
Search result and study characteristics
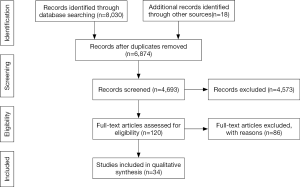
After initial search and removed duplications, a total of 8,202 articles were identified. Eleven cohort (6,11,13,19,31-37), two case-control (12,38), one case-cohort (15) studies for cotton textile workers together with fifteen cohort (39-53), five case-control studies (54-58) for agriculture workers met our criteria and were finally pooled in this meta-analysis. The workflow of study selection was shown (Figure 1).
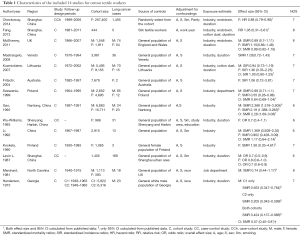
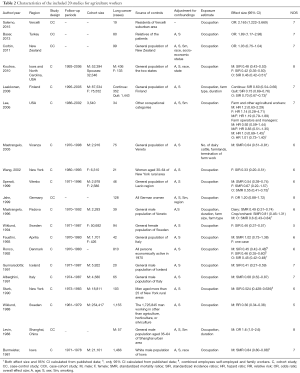
Among the thirty-four accepted study, seven performed in China, seventeen in Europe, seven in USA and three in other countries. All of studies controlled for both age and sex except two (19,54). One study did not provide complete adjusted effect sizes, the other controlled for age and pack-years in their analyses, which did not include lung cancer as an outcome by itself. Therefore, we extracted or calculated crude effect sizes according to original data. Only seven studies adjusted for smoking (12,15,38,41,56-58). Dose-time response were explored in ten studies, of which four quantitatively estimated concentrations of cotton dust or endotoxin exposure (11,15,19,32). Eleven studies stratified according to different exposure duration (11-13,15,19,31,32,38,40,45,58). Detailed characteristics and quality levels of eligible studies were summarized (Tables 1,2).
Full table
Full table
Main, subgroup and sensitive analysis
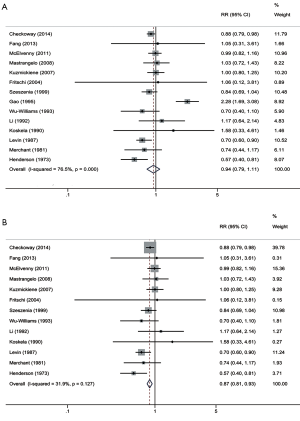
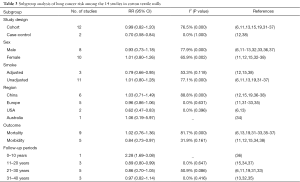
The overall combined RR and corresponding 95% CI of lung cancer in cotton textile mills showed an insignificant result with 0.94 (0.79–1.11) (Figure 2). Substantial heterogeneity was found among studies (I2 76.5%, P=0.000). A publication from China by Gao was probably a great source of heterogeneity as displayed in the Galbraith radial plot and sensitive analysis (Figures 3,4) (36). When we excluded this research and summarized risks of the rest studies, the pooled RR turned out to be a significant result with 0.87 (95% CI, 0.81–0.93), I2 31.9% (P=0.127) (Figure 2). I2 value indicated no heterogeneity was present. In addition, after removing the literature of Gao, the meta-RR was stable regardless of ruling out any of the 13 studies in sensitive analysis. RRs in most subgroup analyses were less than 1.0 (Table 3). The following subgroups showed significant results: case-control study, 0.70 (95% CI, 0.58–0.84); adjusted for smoking, 0.79 (95% CI, 0.66–0.95); USA, 0.62 (95% CI, 0.47–0.83); morbidity as outcome, 0.84 (95% CI, 0.73–0.97); follow up 11–20 years, 0.89 (95% CI, 0.80–0.99).

Full table
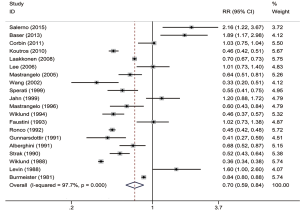
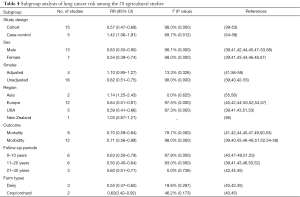
In agricultural investigations, the pooled RR of lung cancer was 0.70 (95% CI, 0.59–0.84) (Figure 5). I2 value exhibited a huge heterogeneity (I2 97.7%, P=0.000). Eight studies deviated slope of the Galbraith radial plot (Figure 3), it was difficult to confirm which studies produced this heterogeneity. According to subgroup analysis (Table 4), heterogeneity was disappeared in four subgroups (adjustment for smoking, Asian countries, follow up over 20 years and farm types), while still presented great in others. Three subgroups showed inverse or insignificant results of reducing lung cancer risk as follows: case-control studies, 1.42 (95% CI, 1.06–1.91); adjusted for smoking, 1.10 (95% CI, 0.95–1.27); Asia region, 1.74 (95% CI, 1.25–2.43). Studies from Europe and USA region had similar meta-RRs with 0.64 (95% CI, 0.51–0.81) and 0.59 (95% CI, 0.41–0.86). Different from the cotton textile studies, the reduction of lung cancer risk was obvious and significant in both male and female subgroups (RR, 0.63, 95% CI, 0.50–0.80 and 0.54, 95% CI, 0.39–0.74, respectively). The lowest risk was found in the follow-up time between 11 to 20 years (RR, 0.50, 95% CI, 0.40–0.64) compared to other two periods. The sensitive analysis was robust (Figure 4).
Full table
Publication bias
There was no evidence of publication bias in either cotton textile studies or agricultural studies according to both Egger’s test (P>0.60) and Begg’s test (P>0.30). Begg’s funnel plots were displayed (Figure 6).
Discussion
This updated meta-analysis indicated that exposure to endotoxin is associated with a 6.0% decreased risk of lung cancer for cotton textile workers, while decreased 30% risk for agriculture workers.
For cotton textile industries, heterogeneity was substantial among the initial 14 studies. A study on Nantong cotton textile workers was the source of heterogeneity (36). This investigation was suggestive of greater lung cancer risks with SMR 2.37 for male and SMR 2.19 for female textile workers. However, these results were based on a 5-year follow-up periods which was much shorter than other investigations. Since exposure to endotoxin was a chronic process, the protective effect of endotoxin was considered to be time dependent. More obvious decreased lung cancer risk has been found in longer durations of employment lasted at least 10 years, even 20 years or more (11,59). Workers at 20–39 age took majority in the Nantong cohort, these young people probably had short durations since first exposure. As follow-up time extended, work years and cumulative endotoxin concentrations would increase correspondingly, the risk of lung cancer among Nantong textile workers perhaps represented a declined trend. Moreover, the cause of death of a few workers were not documented and just speculated according to descriptions of family members, which perhaps led to misclassifications.
A dose-dependent antitumor effect of LPS have been demonstrated in an animal experiment by Morita back in 1996 (60). Several epidemiological researches measured concentrations of endotoxin, showed a wide variation range in cotton mills. Endotoxin levels reduced from early to late stages, ranged from the lowest value 5.8 EU/m3 at packing sit to the highest 10,836 EU/m3 at carding sit in Taiwan textile plants (61). Astrakianakis et al. quantitatively assessed cotton dust and endotoxin concentrations of seven manufacturing processes in three Shanghai textile factories, the geometric mean of endotoxin with the highest levels 1,871 EU/m3 obtained in drawing department and decreased obviously in spinning and weaving departments (17). In our meta-analysis, two of thirteen studies took subgroup discussions according to job departments (13,33). Merchant found a lower lung cancer mortality in yarn processing (SMR 0.3) than in weaving (SMR 0.79). No distinct trends of lung cancer risk among different work sections were observed in Szeszenia’s investigation, perhaps due to mix other synthetic fibers which might increase lung cancer risk (62). Four studies directly measured the concentrations of endotoxin or cotton dust exposure (11,15,19,32). One study by Checkoway showed a modest raised RR in the subgroup of exposure more than 15 years. Compared to the other three, this study with more recent cohort had lower endotoxin concentrations. It was possible that pervasive application of automation equipment and better working environment diminished the exposure, which resulted in greater lung cancer risk. Nearly 70% female workers of Checkoway’s cohort aged over 55 when follow-up began. While this inverse dose-response effect continued more than 10 years but dismissed after 15 years since cessation of exposure (32,63,64). With time went on, workers had more chance to leave industries and stopped exposing. Just as the subgroup of follow-up more than 20 years in our meta-analysis exhibited a protective effect with RR 0.86 (95% CI, 0.73–1.03) and slightly raised in the 31–40 years subgroup. The dose of endotoxin exposure in agriculture work is partly dependent on the types of farming and farm size (65). Many studies have revealed that dairy farmers had more reduction of lung cancer risk compared to the orchard or crop farmers, because of the latter exposed only in the harvested season which less frequent than livestock farmers (40,45). However, similar deficits of lung cancer by farm types or farm size were found in other investigations (66). Compared to crop or orchard farmers, dairy farmers showed a lower cancer risk in the farm types subgroup. Unfortunately, most studies included in our meta-analysis did not classified by type of farming which might be a potential source of heterogeneity.
Smoking is an important confounding factor deserves to be paid more attention in any studies about lung cancer. Smoking in cotton textile industries was prohibited because of an explosion hazard (67). For this reason, previous researches often attributed decreased lung cancer risks to low rate of tobacco smoking. However, after adjusting for smoking, the risk estimate of lung cancer still presented reduction (RR 0.79; 95% CI, 0.66–0.95) for cotton textile workers, but raised in unadjusted group (RR 1.01; 95% CI, 0.80–1.28). Compared to former meta-analysis (18), the reason for this increasing risk had the possibility of insufficient adjustment for residual confounding. It was noteworthy that textile workers in the adjusted group were all from China. Populations in the same region were more likely to have common lifestyle, diet habit, air quality and tobacco varieties. These potential unbalanced baseline could also affect the overall effect sizes, likewise, resulted in heterogeneity. Furthermore, the strongest evidence of increased cancer risk comes from the study conducted by Gao which lack of information about smoking rate. It was still hard to affirm whether death-rates varied little between smokers and nonsmokers exposed to endotoxin.
Relative risk between agricultural work and lung cancer insignificantly elevated with 1.10 (95% CI, 0.95–1.27) in adjustment for smoking subgroup based on four studies. To a degree, farmers in this meta-analysis smoked less than the general population (40,41,47,49,52,54,58,59). A raised meta-RR after controlling for smoking could be predicted. Of note, three of the four studies were case-control studies. Therefore, this slightly elevated RR might emerge from the interaction of adjustment for smoking and study design. A recently published large pooled case-control investigation by Peters et al. demonstrated an increased lung cancer risk with OR 1.13 (95% CI, 1.04–1.22) among farmers (16). Compared with Peters, the pooled relative risk based on five case-control studies was even higher (RR 1.42; 95% CI; 1.06–1.92) in our meta-analysis, which contrary to the cohort subgroup. For a case-control study, memory bias is difficult to avoid. This kind of information bias is influenced by education level and socio-economic status. The insufficient exposure measurement such as farm types perhaps has a degree of misclassification, while the sequence of exposure experience and lung cancer is also hard to judge. Moreover, selection bias is more likely to occur among hospital-based case-control studies. Whether the inadequate correction for smoking in cohort studies or the case-control study design resulted in these inverse outcomes were not clear.
Our research also did not detected large variations of lung cancer risks in different sex for agriculture workers like former meta-analysis demonstrated (18). While for cotton textile workers, we observed that women had more raised summary risk of lung cancer than men. One possible reason was that lung cancer risk connected with endotoxin exposure was considered to be gender differences. Male workers have more chance than female to work in departments involved in high levels of endotoxin (68). The healthy-worker effect, meaning that workers with acute health impairments or severe respiratory syndrome associated with exposure to endotoxin and cotton dust, had intense trend to leave the industry or change job tasks prematurely (69). This effect reduced 10% to 40% mortality rates of workers compared to general populations and then underestimated overall risk ratios (70). It was supposed to have greater influence on male workers than female workers (2,71,72), but was weak in farmers. Since farming was the sole source of incomes, farmers had low rate to quit their work (73). In addition, agriculture workers usually involved in high intensity of physical activities on farm, which were related to the decreased lung cancer risk (74,75).
There were several limitations in our study. First, endotoxin exposure assessment was deficient. Only three studies in our meta-analysis directly measured concentrations of endotoxin, two of them did not provide enough information to draw a dose-response curve, which limited us to identify further association between endotoxin exposure and lung cancer risk. Second, there were still great heterogeneity in agriculture studies, we did not find out source of the heterogeneity. However, the different study design was noteworthy, all of the case-control studies showed obvious increased lung cancer risks, it seems to be inappropriate to explain by exposure misclassification or other biases. Third, because of rare lung cancer incidence and mortality, we took different risk estimates (RR, OR, HR, SMR, SIR) as RR. However, only when the age-specific mortality rates of interest in comparison population are small, the age interval and rang is not too broad can SMR approximate RR. Almost all studies in this meta-analysis have 5-year short age bands, but the age rang has uncontrollability. Some studies involved the elder which might underestimate the RR. Furthermore, SMR is higher than RR in general, the difference usually increases with mortality. Therefore, SMR >1 doesn’t mean the RR is definitely raised (76). Fourth, because of deficient information about smoking histories for participants in most studies, it was still hard to state whether adjustment for smoking would create great influence on the overall risk. Future investigations have the necessary of focusing on the relationship between the dose-time response and lung cancer risk on the basis of sufficient adjustment for smoking and homogeneity.
Conclusions
In conclusion, our findings were consistent with the previous meta-analysis (18). Our investigation added weight to the viewpoint that occupational exposure to endotoxin is inversely associated with lung cancer risk in cotton textile mills and agricultural work.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.05.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liebers V, Raulf-Heimsoth M, Bruning T. Health effects due to endotoxin inhalation Arch Toxicol. 2008;82:203-10. (review). [Crossref] [PubMed]
- Liebers V, Bruning T, Raulf-Heimsoth M. Occupational endotoxin-exposure and possible health effects on humans. Am J Ind Med 2006;49:474-91. [Crossref] [PubMed]
- Rylander R. Endotoxin in the environment--exposure and effects. J Endotoxin Res 2002;8:241-52. [PubMed]
- Lai PS, Christiani DC. Long-term respiratory health effects in textile workers. Curr Opin Pulm Med 2013;19:152-7. [Crossref] [PubMed]
- Michel O, Kips J, Duchateau J, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med 1996;154:1641-6. [Crossref] [PubMed]
- Henderson V, Enterline PE. An unusual mortality experience in cotton textile workers. J Occup Med 1973;15:717-9. [PubMed]
- Andreani V, Gatti G, Simonella L, et al. Activation of Toll-like receptor 4 on tumor cells in vitro inhibits subsequent tumor growth in vivo. Cancer Res 2007;67:10519-27. [Crossref] [PubMed]
- Onier N, Hilpert S, Arnould L, et al. Cure of colon cancer metastasis in rats with the new lipid A OM 174. Apoptosis of tumor cells and immunization of rats. Clin Exp Metastasis 1999;17:299-306. [Crossref] [PubMed]
- Otto F, Schmid P, Mackensen A, et al. Phase II trial of intravenous endotoxin in patients with colorectal and non-small cell lung cancer. Eur J Cancer 1996;32A:1712-8. [Crossref] [PubMed]
- Hodgson JT, Jones RD. Mortality of workers in the British cotton industry in 1968-1984. Scand J Work Environ Health 1990;16:113-20. [Crossref] [PubMed]
- Kuzmickiene I, Stukonis M. Lung cancer risk among textile workers in Lithuania. J Occup Med Toxicol 2007;2:14. [Crossref] [PubMed]
- Levin LI, Gao YT, Blot WJ, et al. Decreased risk of lung cancer in the cotton textile industry of Shanghai. Cancer Res 1987;47:5777-81. [PubMed]
- Merchant JA, Ortmeyer C. Mortality of employees of two cotton mills in North Carolina. Chest 1981;79:6S-11S. [Crossref] [PubMed]
- Lenters V, Basinas I, Beane-Freeman L, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control 2010;21:523-55. [Crossref] [PubMed]
- Checkoway H, Lundin JI, Costello S, et al. Possible pro-carcinogenic association of endotoxin on lung cancer among Shanghai women textile workers. Br J Cancer 2014;111:603-7. [Crossref] [PubMed]
- Peters S, Kromhout H, Olsson AC, et al. Occupational exposure to organic dust increases lung cancer risk in the general population. Thorax 2012;67:111-6. [Crossref] [PubMed]
- Astrakianakis G, Seixas N, Camp J, et al. Cotton dust and endotoxin levels in three Shanghai textile factories: a comparison of samplers. J Occup Environ Hyg 2006;3:418-27. [Crossref] [PubMed]
- Wacholder S, Silverman DT, McLaughlin JK, et al. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol 1992;135:1029-41. [PubMed]
- Fang SC, Mehta AJ, Hang JQ, et al. Cotton dust, endotoxin and cancer mortality among the Shanghai textile workers cohort: a 30-year analysis. Occup Environ Med 2013;70:722-9. [Crossref] [PubMed]
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ 1987;1-406. [PubMed]
- Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale for assessing the quality of nonrandomized studies. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Zhong G, Wang Y, Zhang Y, et al. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 2015;10:e0118333 [Crossref] [PubMed]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1-30. [PubMed]
- Siristatidis C, Sergentanis TN, Kanavidis P, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer--a systematic review and meta-analysis. Hum Reprod Update 2013;19:105-23. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- Galbraitha RF. Graphical Display of Estimates Having Differing Standard Errors. Technometrics 1988;30:271-81. [Crossref]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629-34. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Mastrangelo G, Fadda E, Rylander R, et al. Lung and other cancer site mortality in a cohort of Italian cotton mill workers. Occup Environ Med 2008;65:697-700. [Crossref] [PubMed]
- McElvenny DM, Hurley MA, Lenters V, et al. Lung cancer mortality in a cohort of UK cotton workers: an extended follow-up. Br J Cancer 2011;105:1054-60. [Crossref] [PubMed]
- Szeszenia-Dabrowska N, Wilczynska U, Strzelecka A, et al. Mortality in the cotton industry workers: results of a cohort study. Int J Occup Med Environ Health 1999;12:143-58. [PubMed]
- Fritschi L, Lakhani R, Nadon L. Cancer incidence in textile manufacturing workers in Australia. J Occup Health 2004;46:493-6. [Crossref] [PubMed]
- Koskela RS, Klockars M, Jarvinen E. Mortality and disability among cotton mill workers. Br J Ind Med 1990;47:384-91. [PubMed]
- Gao JL, Zhao LJ, Xu MF. All cause mortality of textile workers in NanTong: a retrospective cohort study (in Chinese). Acta Academiae Medicinae Nantong 1995;2. Available online: http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=6&CurRec=4&recid=&filename=NTYX502.135&dbname=CJFD9495&dbcode=CJFQ&pr=&urlid=&yx=&v=MTUzNTBkcUZTM21VZz09S3puU2RyYTRITS9OcklvcUY1NE9mZ2c1emhBVTRqaDRPWDZUckgwM2ViT1NSYitkWU8=
- Li DH, Zhong YN, Li W, et al. Mortality of cotton and flax textile workers (in Chinese). Chinese Journal of Industrial Medicine 1992. Available online: http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=10&CurRec=5&recid=&filename=SOLE199202004&dbname=CJFD9093&dbcode=CJFQ&pr=&urlid=&yx=&v=MjQzOTMzcVRyV00xRnJDVVJMeWZZdVJvRnl6blY3M1BOaUxIYTdLeEY5UE1yWTlGWUlSOGVYMUx1eFlTN0RoMVQ=
- Wu-Williams AH, Xu ZY, Blot WJ, et al. Occupation and lung cancer risk among women in northern China. Am J Ind Med 1993;24:67-79. [Crossref] [PubMed]
- Koutros S, Alavanja MC, Lubin JH, et al. An update of cancer incidence in the Agricultural Health Study. J Occup Environ Med 2010;52:1098-105. [Crossref] [PubMed]
- Laakkonen A, Pukkala E. Cancer incidence among Finnish farmers, 1995-2005. Scand J Work Environ Health 2008;34:73-9. [Crossref] [PubMed]
- Lee DJ, Fleming LE, Leblanc WG, et al. Occupation and lung cancer mortality in a nationally representative U.S. Cohort: The National Health Interview Survey (NHIS). J Occup Environ Med 2006;48:823-32. [Crossref] [PubMed]
- Mastrangelo G, Grange JM, Fadda E, et al. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol 2005;161:1037-46. [Crossref] [PubMed]
- Wang Y, Lewis-Michl EL, Hwang SA, et al. Cancer incidence among a cohort of female farm residents in New York State. Arch Environ Health 2002;57:561-7. [Crossref] [PubMed]
- Sperati A, Rapiti E, Settimi L, et al. Mortality among male licensed pesticide users and their wives. Am J Ind Med 1999;36:142-6. [Crossref] [PubMed]
- Mastrangelo G, Marzia V, Marcer G. Reduced lung cancer mortality in dairy farmers: is endotoxin exposure the key factor? Am J Ind Med 1996;30:601-9. [Crossref] [PubMed]
- Wiklund K, Dich J. Cancer risks among female farmers in Sweden. Cancer Causes Control 1994;5:449-57. [Crossref] [PubMed]
- Faustini A, Forastiere F, Di Betta L, et al. Cohort study of mortality among farmers and agricultural workers. Med Lav 1993;84:31-41. [PubMed]
- Ronco G, Costa G, Lynge E. Cancer risk among Danish and Italian farmers. Br J Ind Med 1992;49:220-5. [PubMed]
- Gunnarsdóttir H, Rafnsson V. Cancer incidence among Icelandic farmers 1977-1987. Scand J Soc Med 1991;19:170-3. [PubMed]
- Alberghini V, Luberto F, Gobba F, et al. Mortality among male farmers licensed to use pesticides. Med Lav 1991;82:18-24. [PubMed]
- Stark AD, Chang HG, Fitzgerald EF, et al. A retrospective cohort study of cancer incidence among New York State Farm Bureau members. Arch Environ Health 1990;45:155-62. [Crossref] [PubMed]
- Wiklund K, Steineck G. Cancer in the respiratory organs of Swedish farmers. Cancer 1988;61:1055-8. [Crossref] [PubMed]
- Burmeister LF. Cancer mortality in Iowa farmers, 1971-78. J Natl Cancer Inst 1981;66:461-4. [PubMed]
- Salerno C, Carcagnì A, Sacco S, et al. An Italian population-based case-control study on the association between farming and cancer: Are pesticides a plausible risk factor? Arch Environ Occup Health 2016;71:147-56. [Crossref] [PubMed]
- Baser S, Duzce O, Evyapan F, et al. Occupational exposure and thoracic malignancies, is there a relationship? J Occup Health 2013;55:301-6. [Crossref] [PubMed]
- Corbin M, McLean D, Mannetje A, et al. Lung cancer and occupation: A New Zealand cancer registry-based case-control study. Am J Ind Med 2011;54:89-101. [Crossref] [PubMed]
- Jahn I, Ahrens W, Bruske-Hohlfeld I, et al. Occupational risk factors for lung cancer in women: results of a case-control study in Germany. Am J Ind Med 1999;36:90-100. [Crossref] [PubMed]
- Levin LI, Zheng W, Blot WJ, et al. Occupation and lung cancer in Shanghai: a case-control study. Br J Ind Med 1988;45:450-8. [PubMed]
- Agalliu I, Costello S, Applebaum KM, et al. Risk of lung cancer in relation to contiguous windows of endotoxin exposure among female textile workers in Shanghai. Cancer Causes and Control 2011;22:1397-404. [Crossref] [PubMed]
- Morita S, Yamamoto M, Kamigaki T, et al. Synthetic lipid A produces antitumor effect in a hamster pancreatic carcinoma model through production of tumor necrosis factor from activated macrophages. Kobe J Med Sci 1996;42:219-31. [PubMed]
- Su HJ, Chen HL, Huang CF, et al. Airborne fungi and endotoxin concentrations in different areas within textile plants in Taiwan: a 3-year study. Environ Res 2002;89:58-65. [Crossref] [PubMed]
- Hours M, Fevotte J, Lafont S, et al. Cancer mortality in a synthetic spinning plant in Besancon, France. Occup Environ Med 2007;64:575-81. [Crossref] [PubMed]
- Applebaum KM, Ray RM, Astrakianakis G, et al. Evidence of a paradoxical relationship between endotoxin and lung cancer after accounting for left truncation in a study of Chinese female textile workers. Occup Environ Med 2013;70:709-15. [Crossref] [PubMed]
- Mastrangelo G, Rylander R, Cegolon L, et al. Lung cancer risk in subjects exposed to organic dust: an unexpected and surprising story. Thorax 2012;67:1112-author reply 1112-3. [Crossref] [PubMed]
- Basinas I, Sigsgaard T, Kromhout H, et al. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J Expo Sci Environ Epidemiol 2015;25:123-37. [Crossref] [PubMed]
- Blair A, Sandler DP, Tarone R, et al. Mortality among participants in the agricultural health study. Ann Epidemiol 2005;15:279-85. [Crossref] [PubMed]
- Enterline PE, Sykora JL, Keleti G, et al. Endotoxins, cotton dust, and cancer. Lancet 1985;2:934-5. [Crossref] [PubMed]
- Lai PS, Hang JQ, Zhang FY, et al. Gender differences in the effect of occupational endotoxin exposure on impaired lung function and death: the Shanghai Textile Worker Study. Occup Environ Med 2014;71:118-25. [Crossref] [PubMed]
- Bakirci N, Kalaca S, Francis H, et al. Natural history and risk factors of early respiratory responses to exposure to cotton dust in newly exposed workers. J Occup Environ Med 2007;49:853-61. [Crossref] [PubMed]
- McMichael AJ. Standardized mortality ratios and the "healthy worker effect": Scratching beneath the surface. J Occup Med 1976;18:165-8. [Crossref] [PubMed]
- Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med (Lond) 1999;49:225-9. [Crossref] [PubMed]
- Card JW, Carey MA, Bradbury JA, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 2006;177:621-30. [Crossref] [PubMed]
- Armitage TL, Mitchell D, Schenker M. Mortality in the California Farmer Health Study cohort. J Agromedicine 2012;17:288-99. [Crossref] [PubMed]
- Sinner P, Folsom AR, Harnack L, et al. The association of physical activity with lung cancer incidence in a cohort of older women: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev 2006;15:2359-63. [Crossref] [PubMed]
- Thune I, Lund E. The influence of physical activity on lung-cancer risk: A prospective study of 81,516 men and women. Int J Cancer 1997;70:57-62. [Crossref] [PubMed]
- Symons MJ, Taulbee JD. Practical considerations for approximating relative risk by the standardized mortality ratio. J Occup Med 1981;23:413-6. [Crossref] [PubMed]