
Suggested cutoff tumor size for small gastric gastrointestinal stromal tumors
Introduction
Gastrointestinal stromal tumors (GISTs) are the commonest mesenchymal neoplasms of the gastrointestinal tract (1). Based on their phenotypic similarities, GISTs are considered to arise from the interstitial cells of Cajal, the pacemaker cells of the gastrointestinal tract (2). GISTs can occur anywhere throughout the gastrointestinal tract, the most common locations are stomach (60%) and small intestine (30%) (3). Rare cases have been reported in the esophagus, appendix, gallbladder, mesentery, omentum, and retroperitoneum (4).
According to the NCCN guideline (5), gastric GISTs less than 2 cm and with a mitotic index less than 5/50 HPF were considered as very low risk, and conservative follow-up is suggested (6). However, it is believed that small gastric GISTs also have malignant potential, and little is known about the clinical presentation of small gastric GISTs. This makes the decisions on interventions for small gastric GISTs difficult. Previously, we have reported that the mitotic index of 14 out of 63 small gastric GISTs (22.22%) exceeded 5/50 HPF and recommended surgical resection of all small gastric GISTs once diagnosed (7).
Given this situation, we wonder if there is an appropriate cutoff tumor size for small gastric GISTs based on mitotic index, and whether the cutoff size could be used to predict the tumor progression for small gastric GISTs during follow-up.
Methods
Patients
This study was performed in Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical University. From May 2010 to March 2014, a total of 97 patients were enrolled in the present study, including 90 small gastric GIST (≤2 cm) patients underwent resection, and 7 endoscopic ultrasound (EUS) suspected small gastric GIST (1.4–2 cm) patients treated with periodic EUS follow-up according to their own wills. The EUS suspected small gastric GIST was defined as a hypo-echoic tumor arising from the fourth layer of gastric wall on EUS. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from all patients.
Pathology
Specimens were treated routinely for histologic examination in the Department of Pathology in Xijing Hospital of digestive diseases. Immunohistochemistry was performed on 3-μm sections according to the manufacturer’s instructions and the following antibodies: CD117, CD34 and DOG-1. Histological type (spindle, epithelioid or mixed) and mitotic index were also detected by H&E stain.
Follow-up
The EUS suspected small gastric GIST patients who did not receive resection were followed up through EUS and CT every 6 months. Follow-up of the suspected small gastric GIST patients was performed by the same physician in order to avoid measurement bias.
Data collection
Clinicopathological features including age, gender, tumor location, CT enhancement, tumor ulceration, tumor bleeding, tumor size, histological type, mitotic index, NIH risk category, and CD117, CD34 and DOG-1 expression were recorded.
Statistical analysis
Data were processed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Discrete variables were analyzed using the Fisher’s exact test. The optimal cutoff value for small gastric GISTs was determined using the receiver operating characteristic (ROC) curve. The P values were considered to be statistically significant at the 5% level.
Results
Eighteen out of 90 small gastric GISTs had more than 5 mitotic figures per 50 HPF. Since there was a significant proportion of cases with high mitotic index (>5/50 HPF), ROC curve analysis was performed to investigate whether there was an appropriate cutoff size for the prediction of high mitotic index in small gastric GISTs. The result showed that 1.4 cm may be considered as an appropriate cutoff tumor size with a sensitivity of 88.9% and a specificity of 73.6% (Figure 1).
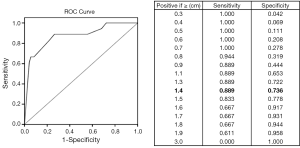
Then, the 90 cases of small gastric GISTs were divided into two groups based on the cutoff tumor size. The clinicopathological features were compared between the two groups and summarized in Table 1. The results showed that age, gender, tumor location, tumor bleeding, histological type, and expression of CD117, CD34 and DOG-1 were comparable between the two groups. However, the ratio of CT enhancement, tumor ulceration, mitotic index exceeds 5/50 HPF and low risk category was significantly higher in tumors between 1.4 and 2.0 cm than that of tumors less than 1.4 cm.

Full table
In order to investigate the risk factors for malignant potential in small gastric GISTs with tumor size between 1.4 and 2.0 cm, correlation between clinicopathological features and mitotic index were analyzed and summarized in Table 2. The results showed that age, gender, tumor location, CT enhancement and tumor bleeding were all comparable between the two groups. The ratio of tumor ulceration was significantly higher in the group with high mitotic index than that with low mitotic index.
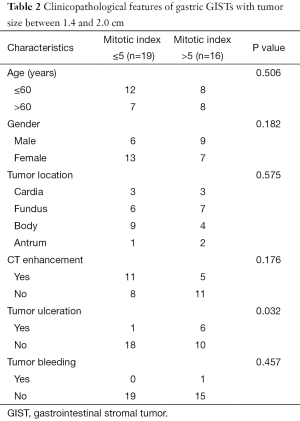
Full table
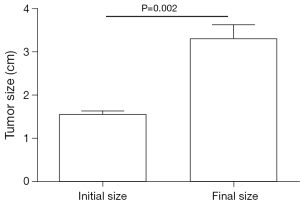
In order to investigate whether the cutoff size could be used to predict tumor progression of small gastric GISTs during follow-up, patients were selected for analysis using the following criteria: (I) suspected as small gastric GISTs through EUS; (II) tumor size between 1.4 and 2.0 cm; (III) without high risk features according to NCCN guideline; (IV) with regular EUS and CT follow-up. Finally, only 7 patients matched the criteria, because most of the patients were lost to follow-up. The median time of follow-up was 11.8 months (range from 10.1 to 24.5 months). The initial and final tumor size was compared. The result showed that the tumor size increased significantly during follow-up (Figure 2, P=0.002).
Discussion
Although the clinical importance of GIST is being increasingly recognized, prediction of the natural course of GIST is still controversial. One problem concerns small gastric GIST. The NCCN guideline recommends that small gastric GIST less than 2 cm can be conservatively followed up (8). However, every GIST is now regarded as potentially malignant, including small gastric GIST. Moreover, it was reported that a small gastric GIST with 1cm in maximal diameter showed rapid growth and early liver metastasis (9). This further highlights the fact that even if the gastric GIST is small, the tumor could show rapid growth, potential for metastasis and poor prognosis.
Previously, we have reported that the mitotic index of 14 out of 63 small gastric GISTs (22.22%) exceeded 5/50 HPF and recommended surgical resection of all small gastric GISTs once diagnosed (7). This indicated that the present cutoff tumor size of 2 cm used to guide the treatment strategy of gastric GIST may be not appropriate to some extent. The present study attempted to find a more appropriate cutoff tumor size for small gastric GISTs based on mitotic index and investigate whether the cutoff size could be used to predict the tumor growth for small gastric GISTs during follow-up.
Mitotic index is the most important prognostic factor for determining the malignant potential of GISTs (10). In our present study, there were approximately 20% of small gastric GIST had more than 5 mitotic figures per 50 HPF, which was consistent with our previous report. Thus, we speculated that there might be a more appropriate cutoff tumor size to predict the malignant potential of small gastric GIST. We found that 1.4 cm may be an appropriate cutoff tumor size associated with mitotic index. Our result was similar with the study reported by Fang et al. (11). They also showed that the best cutoff size associated with tumor progression was 1.4 cm, larger tumor size was associated with tumor progression of small EUS suspected gastric GIST. A study conducted in Israel also investigated the natural history of GISTs, and stated that GISTs larger than 1.7 cm should be monitored by EUS and considered for more aggressive treatment (12). Different from these studies, the cutoff size in our study was calculated based on mitotic index, and the enrolled small gastric GISTs were all confirmed through pathological examination.
Ulceration is one of the risk factors for malignant potential and poor prognosis (13). We found that the ratio of ulceration was significantly higher in tumors between 1.4 and 2.0 cm than tumors less than 1.4 cm. We also found that the NIH risk category could be effectively distinguished by cutoff size of 1.4 cm. These findings indicated that the cutoff size of 1.4 cm could be used to distinguish benign small gastric GISTs from ones with malignant potential.
In order to verify whether the cutoff tumor size of 1.4 cm based on mitotic index could be used to predict the progression of small gastric GISTs or not, tumor progression of small gastric GISTs between 1.4 and 2.0 cm were retrospectively analyzed. Due to the high rate of lost to follow-up, only 7 EUS suspected small gastric GISTs were enrolled in our present study. We found that all of the 7 small gastric GISTs showed significant tumor progression during follow-up. As tumors less than 1.4 cm were not enrolled in the present study, the natural course of these tumors could not be evaluated. At least, the results indicated that small gastric GISTs between 1.4 and 2.0 cm possessed potential of rapid tumor progression during follow-up.
In our present study, the EUS suspected small gastric GISTs were not confirmed by EUS guided fine needle aspiration (EUS-FNA), which was one flaw in our present study and also in the above mentioned studies. It was reported that the diagnostic accuracy of gastric GISTs by EUS alone by experienced endoscopist was 87% (14,15). Thus, the diagnosis of small gastric GISTs only through EUS may affect the results in the present study. EUS-FNA has been recommended for the diagnosis of gastric GISTs between 2 and 5 cm (16). It was reported that the diagnostic sensitivity, specificity and accuracy of EUS-FNA are 66.7%, 100%, and 91.7%, respectively (17). Considering the technical and cost effective points, EUS-FNA was rarely used to assist the diagnosis of small gastric GIST. Thus, the diagnosis of small gastric GIST mainly depends on surgical findings or autopsy (18,19). However, Sekine et al. reported that the sensitivity and positive predictive value of EUS-FNA for small GIST was 81.3% and 100%. Based on EUS-FNA, 15 cases of small gastric GIST increased significantly in tumor size during follow-up (20). The result was consistent with our present study, but more persuasive than our results because their diagnosis of small gastric GIST was confirmed through EUS-FNA. This indicates that EUS-FNA is a highly reliable modality for small GIST.
There are some limitations in our present study. First, it was a retrospective study of a single center’s experience. Multicenter studies are needed to verify the optimal cut off value of small gastric GIST. Second, the sample size was not large enough, which will result in bias during statistical analysis. Third, mitotic index was dichotomized, accurate mitotic index should be identified in the further studies.
Conclusions
In summary, cutoff tumor size of 1.4 cm could be used to distinguish malignant potential for small gastric GISTs. The 1.4 cm may be a more reasonable cutoff tumor size for small gastric GISTs. We recommended that all small gastric GISTs should be resected once diagnosed, at least for tumors between 1.4 and 2.0 cm.
Acknowledgments
Funding: This study was supported in part by grants from the National Natural Scientific Foundation of China (No. 31100643, 31570907, 81572306, 81502403, XJZT12Z03).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xijing Hospital and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mikami T, Nemoto Y, Numata Y, et al. Small gastrointestinal stromal tumor in the stomach: identification of precursor for clinical gastrointestinal stromal tumor using c-kit and α-smooth muscle actin expression. Hum Pathol 2013;44:2628-35. [Crossref] [PubMed]
- Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology 2006;48:83-96. [Crossref] [PubMed]
- Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol 2014;27:S1-16. [Crossref] [PubMed]
- Patil DT, Rubin BP. Gastrointestinal stromal tumor: advances in diagnosis and management. Arch Pathol Lab Med 2011;135:1298-310. [Crossref] [PubMed]
- von Mehren M, Benjamin RS, Bui MM, et al. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012;10:951-60. [PubMed]
- Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:64-7. [PubMed]
- Yang J, Feng F, Li M, et al. Surgical resection should be taken into consideration for the treatment of small gastric gastrointestinal stromal tumors. World J Surg Oncol 2013;11:273. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4.
- Tanaka J, Oshima T, Hori K, et al. Small gastrointestinal stromal tumor of the stomach showing rapid growth and early metastasis to the liver. Dig Endosc 2010;22:354-6. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]
- Fang YJ, Cheng TY, Sun MS, et al. Suggested cutoff tumor size for management of small EUS-suspected gastric gastrointestinal stromal tumors. J Formos Med Assoc 2012;111:88-93. [Crossref] [PubMed]
- Lachter J, Bishara N, Rahimi E, et al. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology 2008;55:1653-6. [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Brand B, Oesterhelweg L, Binmoeller KF, et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis 2002;34:290-7. [Crossref] [PubMed]
- Chak A. EUS in submucosal tumors. Gastrointest Endosc 2002;56:S43-8. [Crossref] [PubMed]
- Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416-30. [Crossref] [PubMed]
- Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc 2002;55:37-43. [Crossref] [PubMed]
- Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 2006;37:1527-35. [Crossref] [PubMed]
- Abraham SC, Krasinskas AM, Hofstetter WL, et al. "Seedling" mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol 2007;31:1629-35. [Crossref] [PubMed]
- Sekine M, Imaoka H, Mizuno N, et al. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc 2015;27:44-52. [Crossref] [PubMed]


