
Primary tracheobronchial mucoepidermoid carcinoma: prognostic factors and EGFR mutation analysis of 33 cases
Introduction
Primary pulmonary mucoepidermoid carcinoma (PMEC) is a rare neoplasm with a frequency of only 0.1–0.2% in primary lung carcinomas (1). PMEC is currently defined as a malignant epithelial tumor, composed of mucus-producing cells as well as intermediate and squamoid cells arranged in solid, glandular or cystic patterns.
PMEC have a wide spectrum of clinical behaviors and all grades of malignancy have been reported. For rarity of its frequency, prognosis of PMEC is not well investigated and no definitive treatment has been established, nowadays. Surgical resection is the main treatment for patients without distant metastasis (2,3). However, the role of chemotherapy and radiotherapy in PMEC is unclear.
Although have showed that the molecular background is similar to those of the salivary gland, the molecular abnormality of pulmonary MEC is not unclear (4-7). Epidermal growth factor receptor (EGFR) is a kind of receptor tyrosine kinase (RTK) that plays an important role in the initiation and development of carcinomas via modulating downstream signaling pathways. The activating mutations located in the tyrosine kinase domains of EGFR could be well-targeted by EGFR tyrosine kinase inhibitors (EGFR-TKIs). Patients with non-small cell lung cancer harboring the EGFR mutations have a well efficacy and low toxicity with EGFR-TKIs treatment (8,9). However, EGFR mutations were not widely detected in PMEC patients for its rarity (10,11).
In the present study, we reviewed a retrospective series of 33 patients with PMEC in Zhuji People Hospital from Jan 1990 to Dec 2013. We emphasized their treatments, and the prognostic factors, and further to evaluate the EGFR mutations status in these patients.
Methods
Patient eligibility
Patients who with pathologically confirmed as PMEC at Zhuji People Hospital from January 1, 1990, to December 31, 2013 were identified. The staging of bronchial PMEC was performed for all patients according to the 7th TNM classification (12). Bhattacharyya’s staging system was adopted in tracheal PMEC (13). The histology of PMEC was according to World Health Organization Classification of Tumors (2004). All cases were graded according to the criteria described by Brandwein et al. (14). The study protocol was approved by the Institutional Review Board of Zhuji people Hospital (zjph-0117) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All of the participants gave informed consent before taking part in the present study.
Statistical analysis
The overall survival was evaluated from the start of treatment to the point of death or last follow-up. Survival curves were calculated according to the Kaplan-Meier method and compared using the log-rank test. Analyses were conducted using the computer software SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Pathology and EGFR mutation examination method
To confirm the histology of PMEC, each of the slides was examined independently by two specialists according to the World Health Organization criteria (2004 version). Immunostains of calponin, collagen IV, CK7, Ki-67, Muc5Ac, p63, p40, and TTF-1 were performed. EGFR mutations analysis was performed using amplification refractory mutation system (ARMS) method with formalin-fixed paraffin embedded archival tissue blocks. The method was according to the manufacturer’s recommendations (Amoy Diagnostics Co., LTD, China).
Results
Patient characteristics
Overall, 33 patients were enrolled in current study. This study group comprised 20 male and 13 female ranging in age from 16 to 75 years (median, 32 years). According the TNM staging system and Bhattacharyya’s staging system, 10 patients were with stage I, 8 with stage II, 9 with stage III, and 6 with stage IV (Table 1). There were 8 patients with histology of low grade, 16 with Intermediate and 9 with high garage based on Brandwein grading system. The clinicopathologic characteristics of all the patients are summarized in Table 1.
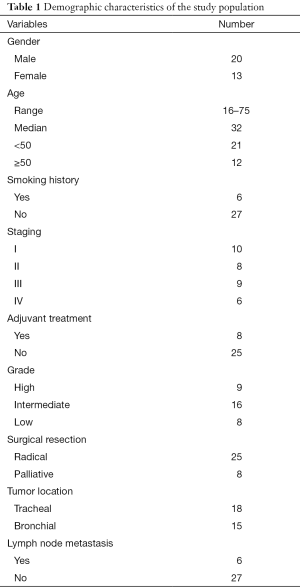
Full table
Pathologic and immunohistochemical findings
Nine patients were with histology of high-grade and 24 with low and intermediate grade. All tumors were composed of mucous, intermediate, and epidermoid cells without keratinization. CK7, Muc5AC, p63, and p40 were positive in all 33 PMEC cases; TTF-1 was negative in all cases. The Ki-67 was ranged from 4% to 70% (mean, 8.5%).
Treatment
Fifteen tumors were located in bronchial and eighteen in tracheal. All of the 33 patients underwent operation, including 25 patients of radical resection and 8 with palliative excision. Twenty-five patients were operated by surgery alone. Eight patients were treated with surgery plus postoperative therapy. Chemotherapy was administered following resection in five of nine patients with high-grade. The regimens comprised of TP (taxol + cisplatin) regimen in three and GP (gemcitabine + cisplatin) in two patients. Two patients received adjuvant radiotherapy after the operation.
Follow-up and prognosis analysis
Median follow-up duration was 141 months (range, 12–211 months). All patients had follow-up data. The 5-year disease-free survival (DFS) and overall survival (OS) rates were 70.9%, 72.3%, respectively. The 5-year OS rate for PMEC patients with high-grade and low-grade tumors were 33.3% and 90.5%, respectively (P=0.001). Stage I and II patients experienced better OS rate than Stage III and IV patients (94.4% vs. 35.8%, P<0.001).Three patients with stage I+II were with high grade, while, six of stage III + IV were with low/intermediate grade. No survival difference was found between the two groups (P=0.480).
Completely resection, stage and histology correlated significantly with DFS and OS (Table 2, Figures 1,2). A multivariate Cox’s regression model was constructed including histologic grade, completely resection and stage as variables. Stage and histology were confirmed as independent prognostic factor for DFS and OS (Table 3).
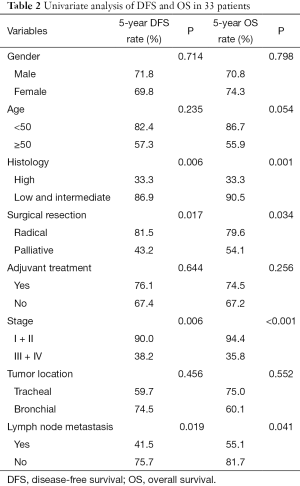
Full table
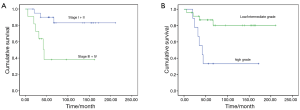
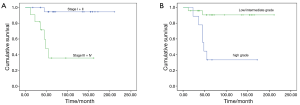
Full table
EGFR mutations in PMEC
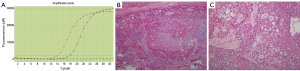
Analysis of EGFR mutation status in 26 of the 33 patients revealed 2 cases (7.7%) with exon 21 L858R mutation (Figure 3A). The first patient with EGFR mutation was a 46-year-old female with histology of high-grade (Figure 3B), and history of no smoking. She received adjuvant chemotherapy with TP regimen after surgery. The other patient was a 29 years old male with histology of low-grade (Figure 3C), and no smoking history. No adjuvant treatment was used in this patient. Both of the two patients were with stage II and no recurrence occurred after surgery, and no EGFR-TKIs were used in these two patients.
Discussion
PMEC was originally described in the salivary glands by Smetana et al. in 1952 (15). For rarity of this tumor, the prognosis and standard treatment are not well defined currently.
Concerning the gender incidence and average age of the patients, experience is not uniform for its rarity. Most of the patients reported for PMEC were with age under 50 years old (16,17). In our series, 21 of the 33 patients were younger than 50 years, with a median age of 32 years. A male predominance was described in several studies (3,18). Twenty cases were male and 13 of female in present study. Only six of our patients were current or ever smokers, which is consistent with previous studies (14).
The clinical behavior of PMEC has been reported to vary from low malignant to highly malignant (19). PMEC is classified as low-grade and high-grade according to the World Health Organization criteria (2004 version). The present study found significantly better survival in patients with low grade histology than in those with high-grade tumors. TNM staging was a significant predictor of prognosis in patients with PMEC (2). The 5-year survival rates were higher in stage I and II patients than stage III and IV. Our results of survival rate between different stage is consistent with previous studies.
Standard treatment for PMEC without metastasis is surgical resection. The surgical procedures including local resection, sleeve resection, lobectomy, segmental resection. For patients with advanced stage, the value of surgery is unclear currently. The role of postoperative treatment is not well investigated. Adjuvant chemotherapy or radiotherapy may be a better choice for patients with advanced stage. In the present study, chemotherapy was administered in five of the eight patients with high-grade histology, however, four of the five patients were with recurrence.
Previous studies showed that EGFR mutations were predominant in female, adenocarcinoma, never-smokers and East Asian NSCLC population. However, EGFR mutations were not widely observed in other solid carcinomas. L858R mutation was detected in two of five PMEC patients in one study (10). There were five patients with exon 21 L861Q mutation in Yu et al. study (11). Two patients were found to harbor EGFR mutations among 26 patients. The targeted therapy may be a promising treatment for some of the PMEC with EGFR mutations through lack of clinical data nowadays.
The major limitations of the present study were its retrospective nature and small number of patients. In addition, a small level of heterogeneity was identified among our patients, which may influence the prognosis analysis. However, for rarity of this carcinoma, our results are considered to be meaningful.
In summary, PMEC is a rare disease. Histological grade and TNM stage were determined to survival. EGFR mutations occurred in some of PMEC patients. It is necessary to validate the prognosis factors and efficacy of EGFR-TKIs in PMEC patients with large number participants.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Zhuji people Hospital (zjph- 0117) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All of the participants gave informed consent before taking part in the present study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yousem SA, Hochholzer L. Mucoepidermoid tumors of the lung. Cancer 1987;60:1346-52. [Crossref] [PubMed]
- Song Z, Liu Z, Wang J, et al. Primary tracheobronchial mucoepidermoid carcinoma--a retrospective study of 32 patients. World J Surg Oncol 2013;11:62. [Crossref] [PubMed]
- Kang DY, Yoon YS, Kim HK, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer 2011;72:250-4. [Crossref] [PubMed]
- Achcar Rde O, Nikiforova MN, Dacic S, et al. Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinoma. Hum Pathol 2009;40:854-60. [Crossref] [PubMed]
- Roden AC, García JJ, Wehrs RN, et al. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol 2014;27:1479-88. [Crossref] [PubMed]
- Huo Z, Wu H, Li J, et al. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One 2015;10:e0143169 [Crossref] [PubMed]
- Zhu F, Wang W, Hou Y, et al. MAML2 rearrangement in primary pulmonary mucoepidermoid carcinoma and the correlation with FLT1 expression. PLoS One 2014;9:e94399 [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Han SW, Kim HP, Jeon YK, et al. Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment. Lung Cancer 2008;61:30-4. [Crossref] [PubMed]
- Yu Y, Song Z, Gao H, et al. EGFR L861Q mutation is a frequent feature of pulmonary mucoepidermoid carcinoma. J Cancer Res Clin Oncol 2012;138:1421-5. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg 2004;131:639-42. [Crossref] [PubMed]
- Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001;25:835-45. [Crossref] [PubMed]
- Smetana HF, Iverson L, Swan LL. Bronchogenic carcinoma; an analysis of 100 autopsy cases. Mil Surg 1952;111:335-51. [PubMed]
- Tsuchiya H, Nagashima K, Ohashi S, et al. Childhood bronchial mucoepidermoid tumors. J Pediatr Surg 1997;32:106-9. [Crossref] [PubMed]
- Yu Y, Song Z, Chen Z, et al. Chinese pediatric and adolescent primary tracheobronchial tumors: a hospital-based study. Pediatr Surg Int 2011;27:721-6. [Crossref] [PubMed]
- Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007;110:2253-9. [Crossref] [PubMed]
- Barsky SH, Martin SE, Matthews M, et al. "Low grade" mucoepidermoid carcinoma of the bronchus with "high grade" biological behavior. Cancer 1983;51:1505-9. [Crossref] [PubMed]


