Surgical organ displacement for proton radiotherapy
Introduction
Proton radiotherapy is a method of applying high-energy particle radiation to treat cancers. Proton therapy is appealing due to its ability to deliver highly conformal dose distributions while minimizing radiation to adjacent normal tissues (1). Proton treatments first began in the 1950s using equipment added on to large nuclear physics labs. The Indiana University Health Proton Therapy Center (IUHPTC, formerly Midwest Proton Radiotherapy Institute) was built at the Indiana University Cyclotron Facility in Bloomington, Indiana, and was the third established center actively treating patients in the United States. At present, there are 10 proton centers treating patients in the US and several more around the world.
In general, the effectiveness of any form of radiation treatment is limited by the tolerances of adjacent normal tissues. The acute and chronic toxicity of radiation (especially to bowel) continues to confound our therapies (2-4). Often the morbidity and cost from the side effects of radiation treatments can become worse than the original disease (5).
While proton radiotherapy treatments can provide more precision in dose delivery, the same problem remains of damage to very closely adjacent structures; conversely, along with a greater ability to provide an intense, targeted dose of radiation to a target volume, there is also the potential to cause greater collateral damage. The authors encountered a number of patients who, despite the accuracy of proton therapy dose distributions, could not be treated adequately and safely due to closely adjacent or abutting vulnerable structures. The question became: can we change the anatomy?
The authors have collaborated in an attempt to surgically alter the patient’s anatomy to make untreatable patients treatable. Here we present our initial series of surgical organ displacements performed on patients with localized cancers of the abdomen and pelvis for whom there were no other acceptable treatment options.
Methods
After obtaining IRB approval, we reviewed the charts of all patients treated at IUHPTC who had had undergone surgical organ displacement with the intention for treatment with proton radiotherapy. We reviewed the pathologic categories, diagnostic images (CT, MRI, and PET), treatment plans and available outcomes of the patients.
Planning and decisions about spacing and strategy were a combined effort of the treatment team. All patients had no evidence of metastatic disease. All patients were initially considered to be untreatable even with protons without some alteration of their anatomy due to adjacent bowel or critical structures preventing sufficient dosage to expect an adequate response. All patients were treated by laparotomy and displacement of organs using omentum and/or saline breast prostheses, anterior oophoropexy, colopexy, or diverting colostomy if required. Multiple metallic fiducial markers were placed at time of surgery to guide treatment planning and for accurate targeting during treatment.
The majority of patients had previous (often multiple) abdominal and pelvic surgical procedures and several had previous conventional radiation treatments to the area as well. There was no assurance given that the displacement procedures would accomplish what was required, and once the procedures were done, the decision to treat was made independently by the radiation oncologist based on the treatment planning CT. Each patient was informed about the uncertainty of adequate displacement and the risk for complications from the displacement procedure themselves, in addition to the risks and benefits of proton radiotherapy.
Our patients ranged from 16 to 82 years of age, and are summarized on Table 1. They included: two recurrent bladder cancers after radical cystectomy, a thrice recurrent desmoid tumor of the rectus sheath, a multiply recurrent desmoid tumor of the retroperitoneum, a previously irradiated and locally recurrent rectal cancer after abdominoperineal resection, a cholangiocarcinoma of the liver, two unresectable hepatocellular carcinomas, an unresectable pelvic sarcoma, a recurrent sacral chordoma, two recurrent pancreatic cancers and an enlarging unresectable cholangiocarcinoma of the liver. In addition, we performed organ displacement for a 21-year-old female with a T3 low rectal cancer, who then underwent subsequent preoperative (neoadjuvant) treatment using protons, and for an 82-year-old with an obstructing carcinoma of the head of the pancreas for whom proton radiation was her only treatment. To our knowledge, the latter two patients are unique in that no such methods to treat malignancies with protons in this way had been previously attempted.
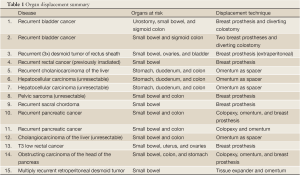
Full table
Results
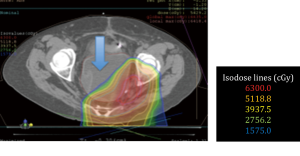
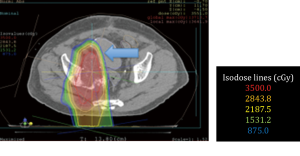
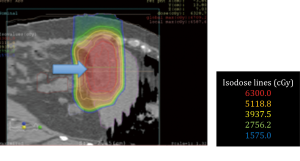
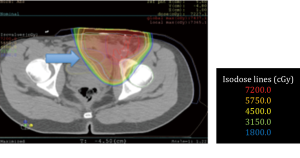
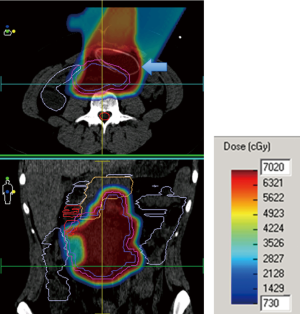
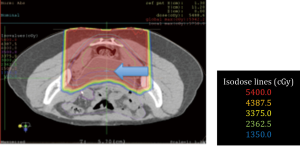
No surgical complications were encountered. All patients obtained adequate displacement to allow for successful proton treatment planning. All completed their treatment course with protons except for one patient with a diagnosis of recurrent pancreatic cancer who developed a perforation of a marginal ulcer at a previous gastrojejunostomy and could not continue; the patient subsequently died of issues unrelated to his radiation or displacement procedure. Two patients had some migration of the spacers that required re-planning; one patient a primary pancreatic cancer, and another with a multiply recurrent retroperitoneal desmoids. Both were able to complete the proton radiation therapy treatment course. Data from this series of patients is shown in Table 1. Selected proton treatment plans are shown in Figures 1,2,3,4,5,6.
All patients who completed their treatment developed no radiation-related complications greater than Grade 2 radiation dermatitis. Mean target dose was 63 Gy(RBE) with a range of 35 to 72 Gy(RBE). The longest follow up is six years in the patient with a pelvic sarcoma. That patient is presently active and without evidence of disease.
The 19-year-old patient with the multiply recurrent retroperitoneal desmoid tumor is 2 years post-radiation and continues to have regression of her tumor. She tolerated 70.0 Gy(RBE) to the abdomen without any GI symptoms during her treatment course nor in the intervening time. She had subsequent removal of the spacer (Figure 5).
The 21-year-old patient who received preoperative proton treatment for a locally advanced rectal cancer after organ displacement completed her abdominoperineal resection and is doing well. She has had no gastrointestinal toxicity and continues to have regular menstrual periods (Figure 6).
The remaining patients are within two years of completion of proton treatment, and none has shown signs of radiation enteritis. Patients displaced for liver tumors have shown complete responses and, at present, have no evidence of disease. All patients with recurrent pelvic tumors have shown good responses to treatment thus far. The one patient who completed proton treatment for recurrent pancreatic cancer was found later to have developed evidence of metastatic disease and is awaiting decisions about further chemotherapy.
Discussion
Surgeons and radiation oncologists have collaborated quite successfully in the treatment of several malignancies. This is most apparent in our present methods of breast conserving therapy for the treatment for breast cancer. Another obvious outcome improvement from collaboration has been in the treatment of patients with locally advanced rectal cancers.
The life-long morbidity of radiation enteritis from conventional radiation treatments has motivated attempts at a variety of surgical displacement procedures in the abdomen, pelvis and retroperitoneum (6-12). Displacement of organs using omentum, tissue expanders, breast prostheses and several types of mesh has been reported, but none of these methods have gained widespread usage (13-16). In addition, techniques like oophoropexy have been employed to move radiosensitive structures out of harm’s way. Despite some apparent advantages, these methods have not entered mainstream clinical practice. For conventional radiation techniques the benefit is often not worth the extra surgical morbidity. The dose to other adjacent structures usually remains high even with displacement.
However, the unique characteristics of proton therapy appeared to present an opportunity in which old ideas might prove to have new and more beneficial applications. Recently, our efforts have been supported by two published case reports demonstrating the utility of displacement methods for proton radiotherapy (17,18).
Proton radiotherapy differs in several important ways from standard external beam radiation therapy. These differences need to be well understood by surgeons and radiation oncologists alike. Conventional therapies use high-energy ionizing radiation that passes through tissues giving both an entrance dose and an exit dose. Therefore, the target volume is normally covered using multiple fields (beam angles) to spread out the entrance and exit doses in order to lessen dosage to normal tissues and to increase the target dose where the beam paths converge.
With protons, which are positively charged particles, the entrance dose tends to be less as most of the protons pass between atomic nuclei near the speed of light. Once into the tissues, the interaction of the protons with the negatively charged electron clouds causes a slowing of the proton trajectories. As the particles slow enough, the inter-nuclear forces bring about an interaction by which the protons impart their energy to the tissues and then stop without an exit dose. This area of high dose is called the Bragg peak. By combining beam energies, this peak can be spread out in depth to cover a larger area that is referred to as the Spread-Out Bragg Peak (SOBP). As the SOBP increases in depth, the entrance dose increases as well (see Figure 7). As with standard radiation methods, multiple beam angles are often required to supply adequate dosage for proton treatments as well.
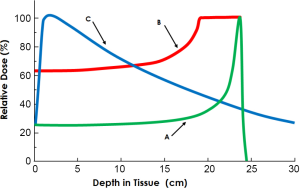
It is critical for surgeons to understand these differences in radiation delivery so that, with organ displacement for proton radiotherapy, one can move critical structures: (I) out of the penumbra of the beam path laterally, (II) beyond the end of the Bragg peak (out of range), and/or (III) proximal in the beam path - out of the high dose Bragg peak. In addition, knowing normal tissue tolerances, effective target dosages, range uncertainties, organ motion issues due to breathing and body motion, dose uncertainties due to air or gas within the tissues, along with constraints of beam angles and beam delivery are all necessary to engage adequately in treatment planning decisions and for proper execution of displacement procedures. This requires significant collaboration between the surgeon and treating radiation oncologist; in several cases, the radiation oncology authors were present at the time of the operation to help determine the displacement technique utilized.
Our series underscores a variety of issues of fundamental importance for treatment of recurrent and unresectable tumors of the abdomen and pelvis using protons. The biology of a specific tumor has a significant impact on our ability to improve survival and palliate symptoms. Adequate and stable displacement in the pelvis is, at present, a promising technique. The upper abdomen remains problematic due to the large number of closely associated organs as well as the motion imparted by the diaphragm. One exception is the liver, for which displacement has been clearly shown, by us and by others, to allow for adequate and safe treatment of liver lesions.
Importantly, evidence of metastatic disease makes these complex pursuits futile in most instances where there can be no significant impact on morbidity and survival. All this can make patient selection for proton radiotherapy treatment challenging.
Logistics can be challenging as well, since patients, at present, often must travel long distances from home for proton treatment at one of the few facilities offering proton radiotherapy. The addition of displacement procedures will lengthen the already lengthy process of proton treatment planning and delivery that can often span several weeks. Additionally, at first, few teams may understand or be accepting of the whole process.
The most important question to ask ourselves about organ displacement for proton radiotherapy is whether or not the risks involved (plus the time and expense) is worth the increased complexity. It is crucial for all involved to understand that the displacement procedure might not work to make proton treatment possible. Plus, the morbidity from the displacement procedure carries significant possible complications itself. We have been most fortunate in this regard thus far.
Previous reports of organ displacement for standard radiation have included complications such as, infections, movement of the spacers, spontaneous deflation or extrusion of a prosthesis, and entero-cutaneous fistulae. Although there have been no reports, concern has been raised about possible impedance of venous return.
Further work in organ displacement using minimally invasive techniques has strong theoretical appeal for use in neo-adjuvant radiation for locally advanced (T3 and greater) rectal cancers and, possibly, for non-metastatic pancreatic cancers. The general feeling in radiation oncology is that if sufficient dosage can be applied in tumors with a high risk of local recurrence, improved outcomes can be expected. We have shown that it is feasible to apply unprecedentedly high doses while improving tissue sparing using proton radiotherapy and surgical organ displacement in both diseases.
In the case of rectal cancers, we have demonstrated that it is possible to displace small bowel and pelvic viscera to completely avoid radiation dosage. We have shown that it is possible to minimize injury to the colon and small bowel and deliver dosages to the pancreas and retroperitoneum [72 Gy(RBE)] that have never been achieved without significant morbidity.
We are presently less enthusiastic about the value of organ displacement for recurrent pancreatic cancers, largely due to our inability to accurately recognize metastatic disease early as well as the often debilitated nature of most of these patients. While there appears to be some hope to lessen local recurrence with our methods used preoperatively, recurrent pancreatic cancer remains a difficult challenge at present.
For patients with recurrent disease after prior radiation therapy, organ displacement - especially of previously irradiated healthy tissue, such as bowel, may facilitate the delivery of an additional course of radiation therapy. However, the morbidity even from an open procedure can be significant in patients that have had previous surgery and radiation; minimally invasive surgery to displace organs for recurrent disease is unlikely to be successful (and may be harmful) because of extensive adhesion formation from surgery, radiation and desmoplastic reactions. In these cases surgical experience and judgment are crucial to bring about satisfactory displacement and minimize morbidity.
The prospect of eliminating, or at least, significantly reducing radiation injury to the bowel is important considering the fact that up to sixty percent of patients receiving pelvic radiation suffer life-long consequences requiring multiple hospitalizations, diagnostic procedures (including further radiation exposure), surgical procedures, loss of work and general misery (5). The cost to the individual and to society from standard pelvic radiation is great. We see opportunity for much improvement.
Conclusions
In our small series of highly selected patients with primary or recurrent pelvic and abdominal tumors, and patients with primary liver tumors, we have convincingly shown that it is technically feasible and safe to alter the anatomical relations in these patients surgically. This can be done with minimal morbidity and convert previously untreatable patients into treatable patients who can receive relatively high doses of radiation with protons.
More significantly, we have shown that it is technically feasible to displace organs to allow neo-adjuvant treatment with particle therapy for locally advanced rectal cancer and pancreatic cancer. These combined techniques hold a double hope of more effectively treating a difficult cancer and also diminishing or eliminating the costly and disabling effects often seen with conventional radiation. We see opportunities for valuable collaboration and innovation in this area, especially in the development of minimally invasive displacement techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Huan Giap and Eric Y Chuang) for the series “Particle Beam Therapy II” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.12.05). The series “Particle Beam Therapy II” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by Institutional Review Board and informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sui H, Urie M. Proton beams in radiation therapy. J Natl Cancer Inst 1992;84:155-64. [PubMed]
- Galland RB, Spencer J. Natural history and surgical management of radiation enteritis. Br J Surg 1987;74:742-7. [PubMed]
- Touboul E, Balosso J, Schlienger M, et al. Radiation injury of the small intestine. Radiobiological, radiopathological aspects; risk factors and prevention. Ann Chir 1996;50:58-71. [PubMed]
- Rubio CA, Jalnas M. Dose-time-dependent histological changes following irradiation of the small intestine of rats. Dig Dis Sci 1996;41:392-401. [PubMed]
- Letschert JG, Lebesque JV, Aleman BM, et al. The volume effect in radiation-related late small bowel complications: results of a clinical study of the EORTC Radiotherapy Cooperative Group in patients treated for rectal carcinoma. Radiother Oncol 1994;32:116-23. [PubMed]
- Shanahan TG, Mehta MP, Bertelrud KL, et al. Minimization of small bowel volume within treatment fields utilizing customized “belly boards”. Int J Radiat Oncol Biol Phys 1990;19:469-76. [PubMed]
- Hindley A, Cole H. Use of peritoneal insufflation to displace the small bowel during pelvic and abdominal radiotherapy in carcinoma of the cervix. Br J Radiol 1993;66:67-73. [PubMed]
- Gallagher MJ, Brereton HD, Rostock RA, et al. A prospective study of treatment techniques to minimize the volume of pelvic small bowel with reduction of acute and late effects associated with pelvic irradiation. Int J Radiat Oncol Biol Phys 1986;12:1565-73. [PubMed]
- Vasilev SA, McGonigle KF, Spencer-Smith EL. Intestinal peritoneal sling as an adjunct to radical pelvic operation and pelvic irradiation. J Am Coll Surg 1995;180:568-72. [PubMed]
- Rodier JF, Janser JC, Roy C, et al. Pelvic exclusion using polyglactin 910 mesh (Vicryl) for preventing radiation injuries of the small intestine Apropos of a series of 24 cases. Bull Cancer 1989;76:1121-5. [PubMed]
- Evans DB, Shumate CR, Ames FC, et al. Use of Dexon Mesh for abdominal partitioning above the peritoneal reflection. Dis Colon Rectum 1991;34:833-5. [PubMed]
- Chen JS. Pelvic peritoneal reconstruction to prevent radiation enteritis in rectal carcinoma. Dis Colon Rectum 1992;35:897-901. [PubMed]
- Sugarbaker PH. Intrapelvic prosthesis to prevent injury of the small intestine with high dosage pelvic irradiation. Surg Gynecol Obstet 1983;157:269-71. [PubMed]
- Burnett AF, Coe FL, Klement V, et al. The use of a pelvic displacement prosthesis to exclude the small intestine from the radiation field following radical hysterectomy. Gynecol Oncol 2000;79:438-43. [PubMed]
- Hong A, Stevens G, Stephen M. Protection of the small bowel during abdominal radiation therapy with a tissue expander prosthesis. Aust N Z J Surg 2000;70:690-2. [PubMed]
- Hoffman JP, Lanciano R, Carp NZ, et al. Morbidity after intraperitoneal insertion of saline-filled tissue expanders for small bowel exclusion from radiotherapy treatment fields: a prospective four year experience with 34 patients. Am Surg 1994;60:473-82; discussion 482-3. [PubMed]
- Fukumoto T, Komatsu S, Hori Y, et al. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J Surg Oncol 2010;101:97-9. [PubMed]
- Komatsu S, Hori Y, Fukumoto T, et al. Surgical spacer placement and proton radiotherapy for unresectable hepatocellular carcinoma. World J Gastroenterol 2010;16:1800-3. [PubMed]