
Clinical trials for charged particle beam therapy
Introduction
After observing the depth-dose properties of protons accelerated by the first cyclotrons designed by Ernest Lawrence in the 1930s, Robert Wilson published in 1946 the seminal paper proposing the use of fast protons for radiation therapy in humans (1,2). Shortly thereafter, Lawrence finished building the 184-inch synchrocyclotron in the Berkeley hills at the future site of Lawrence Berkeley National Laboratory. The so-called “Big Machine” was capable of accelerating both protons and helium nuclei to the much higher kinetic energies needed for the depth of penetration required for human treatment. Since the first patient treated at LBNL in 1954 in the U.S. and at Gustav Werner Institute in Uppsala in 1957, protons and other heavier charged particles have been used increasingly to treat a greater variety of cancers and various nonmalignant conditions. Since then, more than 100,000 patients have been treated with charged particle beam therapy, of which 87,000+ are proton and 14,000 are carbon and other heavier ions (3).
Given the ability to focus the radiation dose conformably on an internal target lesion with less dose to surrounding normal tissues, particle-beam therapy has become more prevalently considered as a better radiation treatment option versus photon therapy. The achievable, 3-dimensional dose-precision with charged-particle irradiation benefits patients by improving local control with a more aggressive dose within the target volume and/or by causing less adverse sequelae to adjacent healthy tissues due to the smaller integral dose outside the target volume.
The technology of proton and heavier-ion therapy has improved clinically over the last few decades. As the therapy is relatively novel, however, standards of treatment for different cancers are currently evolving based upon numerous, ongoing clinical studies. Until the recent compilation of particle-beam-therapy protocols, these studies have been published in a wide variety of medical-science journals. By means of this brief summary, we hope to increase physician awareness of the available clinical trials and of the expanding, evidence-based data worldwide in particle-beam therapy.
Methods
The idea of compiling clinical protocols for proton and heavier-ion therapy was introduced and discussed at the Particle Beam Therapy Co-Operative Group (PTCOG) Publication Committee in May 2011. A standard template was then developed to include the following information: title, principle investigator, contact information, additional information, institution, study purpose, accrual information, primary and secondary aims, methods, eligibility, and exclusion criteria. The presented initiative was subsequently approved by the PTCOG Steering Committee.
The collection of clinical trials was accomplished by auditing the National Cancer Institute (NCI) www.clinicaltrials.gov website and through Steering Committee members (4). All radiation treatment protocols found were then individually examined, and the relevant information for all charged-particle studies was entered into the previously developed, formatted templates for tabulation and compilation. Any missing information at participating institutions worldwide was gathered by email inquiry or left blank. All protocols were then organized by treatment site. Subsequently, the compilation was reviewed by PTCOG for accuracy and completeness. Additional protocols will be added as they are initiated.
Results
A total of 64 protocols were compiled from those available on the NCI website, from PTCOG members, and from email inquiries, representing the efforts of ten proton and/or heavier-ion therapy centers. The following tables summarize the compilation overview, list the participating institutions, and highlight the treatment sites and categories currently under investigation for response to charged-particle therapy.
Discussion
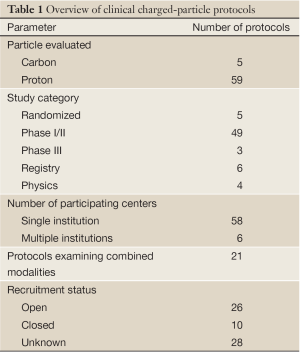
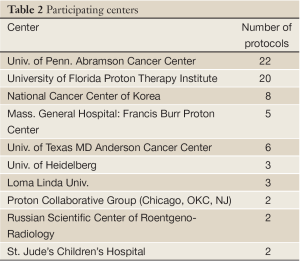
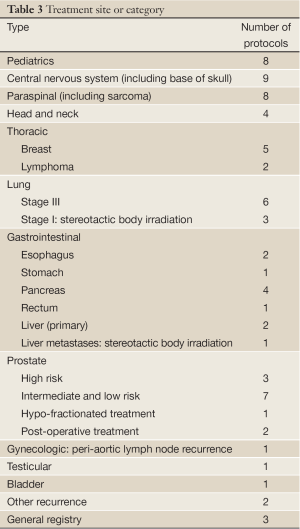
Table 1 lists 64 clinical protocols underway in 10 institutions. Proton therapy is being studied in 59 trials and carbon therapy in 5 trials. Multi-modality treatments (chemotherapy and also including X-ray therapy) are evaluated in 21 protocols. The majority are phase I/II studies, but 5 are prospective, randomized trials (three comparing proton versus carbon). Six have multi-center collaboration, and the rest are of single-institution variety. Table 2 lists the number of clinical trials at each of the 10 centers, and Table 3 lists them by disease site. The majority of trials are in the prostate [13], pediatric [8], CNS [9] and para-spinal [8] areas, but there are also a good number involving breast [5] and lung [9]. For the breast studies, proton beam therapy is used in boost treatment and also in accelerated partial breast irradiation. For lung studies, proton beam therapy is used in treatment of advanced (stage III) lung cancer and also in early stage (I) as hypofractionation or as stereotactic body radiation therapy. For GI sites, proton beam therapy is used in variety of sites including liver, pancreas, rectum and esophagus.
Full table
Full table
Full table
Conclusions
Despite that more than 100,000 patients have been treated with particle beam over the last 60 years at more than 30 centers around the world, only a small percentage of these patients are treated on clinical trials. As increasing number of particle beam centers are in operation and being developed, the clinical indications for particle beam therapy need to go beyond the traditional uses such as pediatrics, ocular tumors, sarcoma, and base of skull tumors. The compiled list of clinical protocols shows a diversified potential applications in cancers of the lung, head and neck, gastrointestinal tract, prostate, breast, brain, gynecologic sites, lymphoma, and recurrent tumors. These clinical trials will validate or invalidate the use of particle beam for these disease sites. The compilation and posting of clinical trials will enhance awareness and accelerate the patient accrual to provide the answers. The other benefit of these listings will be for clinicians who are planning new clinical trials basing on these information, and we hope to promote multi-center collaboration. The authors believe that likely there may be more, now-unreported clinical trials currently underway, and we hope that these centers will recognize our efforts and contribute to this listing.
Acknowledgments
The authors wish to acknowledge members of the PTCOG for their assistance in compiling the data presented. The authors are also grateful to the 10 particle beam centers (listed in Table 2) who develop these clinical trials and contribute their time and effort to this listings.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Particle Beam Therapy II”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2012.12.06). The series “Particle Beam Therapy II” was commissioned by the editorial office without any funding or sponsorship. HBG served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Translational Cancer Research. RPL serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson RR. Radiological use of fast protons. Radiology 1946;47:487-91. [PubMed]
- Suit HD, Chu W. History of Charged Particle Radiotherapy. In: Pine JW Jr. eds. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins, 2008:1-7.
- Available online: www.ptcog.web.psi.ch accessed on Jan 14, 2013.
- Available online: www.clinicaltrials.gov


