
Clinical model to predict progression-free survival in EGFR-mutant lung adenocarcinoma patients treated with first-generation EGFR-TKIs
Introduction
The discovery of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) is a landmark event for survival improvement of non-small cell lung cancer (NSCLC) patients (1). In early studies, gefitinib and erlotinib showed promising efficacy in unselected NSCLC patients. However, differences in outcome were soon recognized after receiving these drugs. Subsequent clinical studies revealed that factors, such as East-Asian origin, female sex, adenocarcinoma histology, a non-smoking history were found to be predictors for a favorable response to EGFR-TKIs in unselected NSCLC (2-4). Mechanistic studies demonstrated that the above characteristics were more likely found in patients harboring specific mutations in the tyrosine kinase domain of EGFR (5-8). At the same time, these mutations were found to be oncogenic driver mutations by two study groups (1,6). Exon 19 deletions (19del) and a point mutation in exon 21 (21L858R) were the most commonly found EGFR mutations, comprising approximately 85% of lung-cancer-specific EGFR sensitive mutations (9).
Gefitinib showed a significant higher response and longer progression-free survival (PFS) compared with traditional chemotherapy (10). Based on the evidence, EGFR-TKIs are now recommended as a first-line treatment for NSCLC patients harboring EGFR sensitive mutations (11,12).
EGFR mutation status is widely acknowledged as the best predictor for EGFR-TKIs efficacy. Albeit it may be superior to other clinical and pathological factors for predicting response to EGFR-TKIs (13,14), the efficacy might differ a lot in patients with the same EGFR sensitive mutations (15). Thus, exploring factors associated with EGFR-TKIs efficacy other than EGFR mutation status is vital, especially for patients with EGFR-activating mutations.
In the current retrospective research, we first collected and analyzed clinicopathologic data on Chinese lung adenocarcinoma patients harboring EGFR-activating mutations after they were treated with EGFR-TKIs to identify independent factors of PFS, and then established a predictive model to further discover the implications of these factors.
Methods
Patients
The present study collected data on a total of 128 patients with EGFR-activating mutations who received first-generation EGFR-TKIs (including gefitinib, erlotinib or icotinib) between July 1, 2010 and December 1, 2013 at the Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University. All patients were diagnosed as lung adenocarcinoma with IIIB or IV stages according to the TNM system set by the International Association for the Study of Lung Cancer (IASLC). Patients with symptomatic brain metastases, an Eastern Cooperative Oncology Group performance status (ECOG PS) of more than 2, or with missing data were not included in the study. Those who received sequential chemotherapy during the course of targeted therapies were also excluded.
Clinical factors, such as age, sex, smoking history, EGFR mutation sites, clinical stages, metastatic sites, pulmonary surgical history, tumor differentiation, tumor locations, pretreatment (within 2 weeks) levels of six serum tumor markers [including carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cancer antigen 125 (CA125), squamous cell carcinoma (SCC) antigen, cytokeratin-19 fragments (CYFRA21-1), and lactate dehydrogenase (LDH)], ECOG PS, and treatment timing with EGFR-TKIs were all collected and analyzed, along with the patients’ PFS times.
This study was approved by the Ethics Committee of Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai. The Approval Number is K(P)15-04. The participants of the present study did not write informed consent before taking part since this was a retrospective study.
Detection of EGFR and serum tumor markers
ADx EGFR Mutation Detection Kit (Amoy Diagnostics, Xiamen, China), which has been approved by China’s Food and Drug Administration (CFDA) was used to detect EGFR mutations. Serum tumor markers were detected by radioimmunoassay. The cut-off values for levels of CEA, NSE, CA125, SCC, CYFRA21-1 and LDH were: 5 ng/mL, 25 ng/mL, 35 U/mL, 1.5 µg/L, 5 ng/mL, and 250 U/L, respectively.
Administration of EGFR-TKIs, response assessment and follow-up
Gefitinib and erlotinib were administered in dosages of 250 and 150 mg once daily, respectively, while icotinib was administered in a dosage of 125 mg 3 times daily. All patients received 1 of the 3 EGFR-TKIs in a 28-day cycle.
All patients were evaluated by computed tomography (CT) of the thorax to acquire tumor baseline information before the administration of EGFR-TKIs. Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 after the first cycle of therapy and subsequently after every two cycles. Routine thorax CT scan and abdominal ultrasound was carried out every time a patient came to follow-up. Bone scan and enhanced magnetic resonance imaging (MRI) of the brain was also performed when necessary. The cutoff date for the study was April 1, 2015.
Statistical analysis
All statistical analyses in this study were performed using SPSS® software, version 13.0 (SPSS Inc., Chicago, IL, USA). PFS was calculated as the time from the date EGFR-TKIs were first administered until the date of discontinuation or until the death of a patient.
Firstly, a Kaplan-Meier method and log-rank tests were used to analysis PFS. Factors with P values no more than 0.05 in different levels were selected to entera Cox proportional hazards model to identify the independent prognostic factors associated with PFS. Subsequently, a prognostic index (PI) model was generated according to the results of Cox regression analysis. Finally, each patient of the study was calculated a PI based on the model, and then was divided into different groups according to the quartiles of PI to further compare PFS using log-rank tests and a pairwise over strata method.
All confidence intervals reported in the present study were 2-sided, and P values no more than 0.05 were considered statistically significant.
Results
Patient characteristics and response assessment
Table 1 summaries the baseline characteristics of the 128 lung adenocarcinoma patients collected in the present study. These patients tended to be young (<60 years of age, 57.8%), female sex (61.7%), non-smokers (71.1%) and stage IV (91.4%). Sixty-five patients (50.8%) harbored EGFR 19del while 63 (49.2%) harbored a 21L858R mutation. The numbers of patients who received gefitinib, erlotinib, and icotinib were 69 (53.9%), 22 (17.2%) and 37 (28.9%), respectively.

Full table
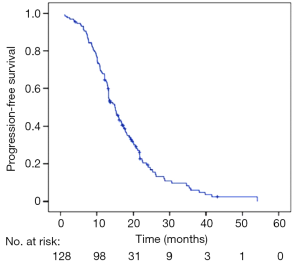
At the study cutoff date, 110 of the 128 patients (85.9%) discontinued EGFR-TKIs therapy, while 18 (14.1%) did not stop taking EGFR-TKIs. The median PFS for all 128 patients was 14.9 months (95% CI, 13.2–16.5 months) (Figure 1).
Univariate survival analysis
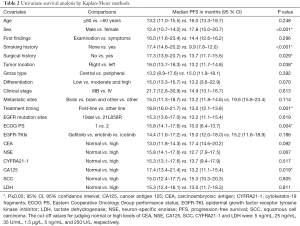
Table 2 shows the results of the univariate survival analysis by the Kaplan-Meier method. The analyses suggested that female sex (PFS 17.8 vs. 12.4 months for males; P<0.001), a non-smoking history (PFS 17.4 vs. 9.9 months for a history of smoking; P<0.001), a surgical history of lung cancer (PFS 17.3 vs. 13.7 months for no surgical history; P=0.029), tumor located in the right lung (PFS 16.0 vs. 13.2 months for the left lung; P=0.038), a first-line EGFR-TKIs administration (PFS 18.9 vs. 13.0 months for other lines; P=0.001), ECOG PS 1 (PFS 15.8 vs. 10.0 months for PS 2; P=0.004), and a normal pretreatment CA125 level (PFS 17.4 vs. 13.2 months for a high level; P=0.019) were all predictors of a longer PFS. No statistically significant differences in PFS were found for age, first findings, tumor gross type, tumor differentiation, clinical stages, metastatic sites, EGFR mutation sites, EGFR-TKIs, and pretreatment serum levels of CEA, NSE, CYFRA21-1, SCC and LDH.
Full table
Multivariate regression analysis and modeling
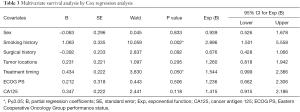
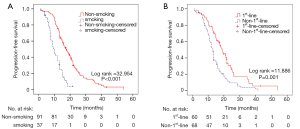
Table 3 lists the outcomes of multivariate survival analysis by Cox regression methods. A non-smoking history [hazard ratio (HR), 2.896; 95% CI, 1.501–5.558; P=0.002] and first-line EGFR-TKIs treatment (HR, 1.544; 95% CI, 0.999–2.386; P=0.05) were found to be independent predictive factors of a longer PFS with EGFR-TKIs therapy. However, other factors including sex, surgical history, tumor locations, ECOG PS and pretreatment CA125 levels were not independent predictors of PFS. The PFS curves regarding smoking history and treatment timing are showed in Figure 2.
Full table
Based on the results of Cox regression, a PI model can be established as: PI =1.063 × Smoking + 0.434 × Timing. In our subsequent analysis, value assignments of smoking and treatment timing were defined as: for smoking, 1= non-smoking, 2= smoking; for treatment timing, 1= first-line therapy, 2= non-first-line therapy.
Further analysis according to the established PI model
Each patient was calculated a PI according to the above model. The 25%, 50% and 75% quartiles of PI were 1.479, 1.931, and 2.560, respectively. Firstly, we divided patients into four groups according to the quartiles. As the model contains two factors with two levels each, the four groups were group A (PI =1.497), group B (PI =1.913), group C (PI =2.560) and group D (PI =2.994). The implication of each group has been listed in Table 4. Table 5 and Figure 3A shows PFS comparisons of the four groups. Generally, there is a significant statistical difference among four groups in PFS. However, pairwise comparisons regarding PFS of different groups suggested that there was no statistical difference in two comparisons: group B and group C, group C and group D.

Full table

Full table
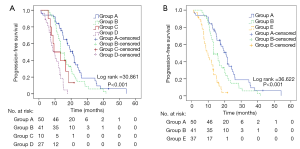
As we considered the reason might be due to the small sample size of group C, we merged group C and group D into group E (PI ≥2.560). Then the statistical analysis was repeated. The implication of group E is “smoking regardless of treatment timing” (Table 4). Table 5 and Figure 3B shows PFS comparisons of the three groups. Pairwise comparisons regarding PFS suggested that statistical difference existed in group A, group B and group E indicating that there were differences between the three groups in PFS: non-smoking and first-line therapy, non-smoking and non-first-line therapy, smoking regardless of treatment timing.
Discussion
In this study, we found that a non-smoking history and first-line EGFR-TKIs treatment were independent predictive factors of a longer PFS with EGFR-TKIs therapy by analyzing the data in our institute. According to the results of Cox regression analysis, a predictive model was established as PI =1.063 × Smoking + 0.434 × Timing. Based on the model, we further discovered the PFS differences among the three groups: non-smoking and first-line therapy, non-smoking and non-first-line therapy, smoking regardless of treatment timing.
Existing data showed that never smokers might have a favorable response to EGFR-TKIs since these patients are more likely to harbor EGFR sensitive mutations than ever smokers (16,17). Interestingly, in patients with EGFR-activating mutations, smoking was also a factor related to EGFR-TKIs efficacy. Our study indicated that a non-smoking history was an independent predictor of a longer PFS in EGFR-mutant lung adenocarcinoma patients treated with EGFR-TKIs, which is consistent with previous findings (18). However, we did not analyze the impact of smoking dosages due to the small sample size of our study. It has been reported that smoking dosage of more than 30 pack-years is an independent predictor for poor efficacy of EGFR-TKIs in EGFR-mutant lung adenocarcinoma patients (15). Specific molecular mechanisms about this phenomenon remain unknown. Some possible explanations include cigarette smoking-induced EGFR post-translational changes, activation of the nicotinic acetylcholine receptor, promotion of EGFR signal or epithelial-mesenchymal transition (19-22).
As we know, first-line EGFR-TKIs therapy in patients with EGFR-activating mutations achieves a longer PFS, compared with first-line standard chemotherapy (23-25). Previous data revealed that response rate of first-line gefitinib treatment was higher than that of non-first-line patients; however, there is no difference in OS (26). Our data showed that first-line EGFR-TKIs treatment was an independent predictor of a longer PFS. However, this result should be interpreted with caution since P value for comparison of different levels in treatment line was just 0.05. To date, views on treatment timing of EGFR-TKIs are not completely uniform albeit EGFR-TKIs are recommended as a first-line therapy. However, numerous opinions, including assurance on drug exposure, improvement in quality of life, better tolerance by patients with poor PS support the general application of first-line EGFR-TKIs (27).
It has been reported that pretreatment serum tumor markers are associated with EGFR-TKIs efficacy in patients with EGFR sensitive mutations. Higher pretreatment CEA levels may be associated with a worse outcome in EGFR-mutant patients treated with first-line EGFR-TKIs (28). Low pretreatment CYFRA21-1 levels were found to be an independent favorable predictor for longer OS in lung adenocarcinoma patients with EGFR mutations (29). In addition, EGFR-mutant NSCLC patients with an elevated serum NSE level might have significantly shorter PFS and OS after EGFR-TKIs therapy (30). However, our study did not show these serum tumor markers could influence the PFS of EGFR-TKIs in EGFR-mutant Chinese lung adenocarcinoma patients. The design of the previous studies differed from our study, and many studies with small sample size, and were retrospectively conducted in NSCLC patients instead of lung adenocarcinoma patients. We hypothesized that these factors might affect the outcomes.
In multivariate regression analysis, sex, surgical history, tumor locations, ECOG PS and pretreatment CA125 levels were not independent predictors of PFS although these factors could affect PFS in univariate analysis by Kaplan-Meier methods. The possible reason may be that these factors are more important as prognostic factors rather than predictive factors in EGFR-mutant lung adenocarcinoma patients receiving EGFR-TKIs (31).
The predictive model established in the study: PI =1.063 × Smoking + 0.434 × Timing can be used as a reference for clinical practice. By further analyzing the model, we found that patients with a smoking history had a shorter PFS regardless of treatment timing. The results of Cox regression analysis and PI model indicated that smoking might affect PFS more than treatment timing. Thus, EGFR-mutant lung adenocarcinoma patients who have a smoking history are more likely to acquire a relatively short PFS according to our findings.
This study has some limitations. First of all, many confounders might be inevitably introduced to the study due to its retrospective nature. For example, three EGFR-TKIs were not randomly assigned, but allocated mainly as the physicians’ recommendation. Side effects were not collected in this study for its retrospective nature, but previous study has indicated that skin rash might be a predictor of erlotinib efficacy (32). Secondly, as a single center study, EGFR-TKIs might show larger treatment efficacy than multicenter studies. Moreover, the sample size of our study was relatively small, especially when we use these data to establish the PI model. Last but not least, the model we established should be interpreted with caution since P value for treatment timing was just 0.05 in Cox analysis.
In conclusion, findings of the present study suggest that a non-smoking history and a first-line EGFR-TKIs treatment timing are independent predictors of a longer PFS in EGFR-mutant lung adenocarcinoma patients treated with first-generation EGFR-TKIs. PFS is longer for those who are never smokers and receive first-line EGFR-TKIs, compared with other groups. However, taking the limitations of this study and the importance of exploring factors associated with EGFR-TKIs efficacy in EGFR-mutant patients into account, subsequent prospective analyses with larger sample sizes are needed to confirm the results.
Acknowledgments
Funding: This project was supported by the National Nature Science Foundation of China (Grant No. 81472175) and Shanghai Municipal Commission of Health and Family Planning Key Projects (Grant No. 20134007).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai. The Approval Number is K(P)15-04. The participants of the present study did not write informed consent before taking part since this was a retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Haringhuizen A, van Tinteren H, Vaessen HF, et al. Gefitinib as a last treatment option for non-small-cell lung cancer: durable disease control in a subset of patients. Ann Oncol 2004;15:786-92. [Crossref] [PubMed]
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer–molecular and clinical predictors of outcome. N Engl J Med 2005;353:133-44. Erratum in: N Engl J Med 2006;355:1746. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004;64:8919-23. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839-44. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Azzoli CG, Temin S, Aliff T, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol 2011;29:3825-31. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [PubMed]
- Toyooka S, Takano T, Kosaka T, et al. Epidermal growth factor receptor mutation, but not sex and smoking, is independently associated with favorable prognosis of gefitinib-treated patients with lung adenocarcinoma. Cancer Sci 2008;99:303-8. [Crossref] [PubMed]
- Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 2005;11:1167-73. [PubMed]
- Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014;84:196-202. [Crossref] [PubMed]
- Ahn MJ, Park BB, Ahn JS, et al. Are there any ethnic differences in molecular predictors of erlotinib efficacy in advanced non-small cell lung cancer? Clin Cancer Res 2008;14:3860-6. [Crossref] [PubMed]
- Tomizawa Y, Iijima H, Sunaga N, et al. Clinicopathologic significance of the mutations of the epidermal growth factor receptor gene in patients with non-small cell lung cancer. Clin Cancer Res 2005;11:6816-22. [Crossref] [PubMed]
- Zhang Y, Kang S, Fang W, et al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer 2015;16:144-151.e1. [Crossref] [PubMed]
- Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther 2012;11:795-804. [Crossref] [PubMed]
- Wang S, Takayama K, Tanaka K, et al. Nicotine induces resistance to epidermal growth factor receptor tyrosine kinase inhibitor by α1 nicotinic acetylcholine receptor-mediated activation in PC9 cells. J Thorac Oncol 2013;8:719-25. [Crossref] [PubMed]
- Li H, Wang S, Takayama K, et al. Nicotine induces resistance to erlotinib via cross-talk between α 1 nAChR and EGFR in the non-small cell lung cancer xenograft model. Lung Cancer 2015;88:1-8. [Crossref] [PubMed]
- Liu M, Zhou C, Zheng J. Cigarette smoking impairs the response of EGFR-TKIs therapy in lung adenocarcinoma patients by promoting EGFR signaling and epithelial-mesenchymal transition. Am J Transl Res 2015;7:2026-35. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Chang GC, Tsai CM, Chen KC, et al. Predictive factors of gefitinib antitumor activity in East Asian advanced non-small cell lung cancer patients. J Thorac Oncol 2006;1:520-5. [Crossref] [PubMed]
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J Clin Oncol 2013;31:1081-8. [Crossref] [PubMed]
- Chen YM, Lai CH, Chang HC, et al. Baseline, Trend, and Normalization of Carcinoembryonic Antigen as Prognostic Factors in Epidermal Growth Factor Receptor-Mutant Nonsmall Cell Lung Cancer Patients Treated With First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Medicine (Baltimore) 2015;94:e2239 [Crossref] [PubMed]
- Ono A, Takahashi T, Mori K, et al. Prognostic impact of serum CYFRA 21-1 in patients with advanced lung adenocarcinoma: a retrospective study. BMC Cancer 2013;13:354. [Crossref] [PubMed]
- Inomata M, Hayashi R, Yamamoto A, et al. Plasma neuron-specific enolase level as a prognostic marker in patients with non-small cell lung cancer receiving gefitinib. Mol Clin Oncol 2015;3:802-6. [PubMed]
- Yang CH, Yu CJ, Shih JY, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol 2008;26:2745-53. [Crossref] [PubMed]
- Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J Clin Oncol 2004;22:3238-47. [Crossref] [PubMed]


