
Personalized molecular targeted therapy for hepatocellular carcinoma in the era of genome sequencing
Nowadays, hepatocellular carcinoma (HCC) represents a major health problem worldwide, being the second most frequent cause of cancer-related deaths (1). Among the different cancer types, HCC is the fifth most common in men and the seventh in women (1). In the liver, HCC is the first primary malignancy, accounting for about 90% of hepatic cancers. Its incidence is globally increasing, exceeding the 780,000 new cases in 2012 (2,3). Because of its high lethality, incidence and mortality of HCC are almost equal (1-3). Albeit with a global diffusion, HCC hits hard in some specific geographic areas, since over 80% of cases are diagnosed in the Sub-Saharan Africa and in East Asia, in contrast with North and South America, North Europe and Oceania, where HCC incidence is lower, whereas in the Mediterranean countries HCC risk is intermediate (4). Notably, although incidence of HCC progresses with age, reaching a peak between 60 and 75 years, its detection in young individuals is steadily increasing in the last decade, thus enhancing its socio-economic impact (4). In nearly 90% of cases, HCC develops on a background of chronic liver disease, generally cirrhosis (5,6), where the frequency of tumor development increases over the course of the disease (3.9% annual incidence), and HCC is actually the leading cause of death in patients with cirrhosis related to HCV infection (7). To date, a wide range of options can be offered for the treatment of these patients, including surgical resection, liver transplant, percutaneous ablation, transarterial chemoembolization, depending upon the residual hepatocellular function, and the tumor development (size and stage). Despite this apparent richness of treatments, there is a startling lack of therapeutic effectiveness as the overall 5-year survival of HCC remains low (<20%), due to the severe dysfunction related to cirrhosis, the marked tumor aggressiveness, or even, the late diagnosis (8). In fact, there is still a high proportion of patients (up to 70–90% in Western countries and in Asia, respectively) (9), where regrettably, HCC is diagnosed at an already advanced stage, and thus, patients are eligible only for palliative therapies. At this stage, only one approved systemic therapy is available, sorafenib, which was introduced in the treatment algorithm of HCC nearly ten years ago (10,11). Sorafenib is a multi-tyrosine kinase inhibitor acting on both tumor cell proliferation and tumoral neoangiogenesis. The pleiotropic anti-tumoral activities exerted by sorafenib, depend upon its ability to target the Raf kinases (B-Raf, the oncogenic B-Raf V600E, and C-Raf), a key regulatory point of the mitogen-activated protein kinases (MAPK/ERK) pathway, and to inhibit the activation of several receptors, including the vascular endothelial growth factor receptors-1, -2, and -3 (VEGFR1–3), the platelet-derived growth factor receptor-β (PDGFRβ), and the stem cell growth factor receptor c-Kit (CD117) (12). Unfortunately, after an initial enthusiasm, sorafenib failed to live up the expectations as shown in several following studies. In fact, the SHARP study showed in treated patients, a slight increase in the overall survival (3 months) concomitant with a reduced radiologic progression compared with placebo (11). Moreover, the STORM trial, a phase III study, aimed at evaluating the efficacy of sorafenib as adjuvant treatment following liver resection or local ablation, demonstrated that it did not ameliorate the overall survival and the disease free survival from the first therapeutic intervention (13). Although with limited favorable results, sorafenib represents indeed a breakthrough in the HCC treatment, because it paves the way for a novel molecular-based approach targeting fundamental mechanisms of cancer invasiveness. Starting from the sorafenib experience, several clinical trials were then designed to test drugs and small molecules capable to specifically inhibit the signaling pathways known to be deregulated in HCC. In particular, a number of studies were first performed to test compounds able to interfere with the signal pathways activated by VEGF and PDGF, pivotal players of the tumoral neoangiogenesis dictating the early metastatic spread of HCC. Based on data derived from other epithelial tumors, such as ovary and breast cancer (14,15), the last years witnessed a general burst of translational studies testing a variety of molecular targets. Among them, some tyrosine kinases, including the receptors for the epidermal growth factor (EGFR), the fibroblast growth factor (FGFR), or the hepatocyte growth factor (cMet), the TGFβ signaling, or some common downstream effectors, such as MAPK/ERK, or the mammalian target of rapamycin (mTOR), have been investigated. Unfortunately, results from these studies have been largely disappointing, showing no improving effects with respect to sorafenib or even, to the other more conventional chemotherapeutic regimens. However, the failure of these strategies should reflect the huge genetic heterogeneity featuring HCC where multiple mutations in several key oncogenic drivers are variably involved depending on the etiology of the underlying cirrhosis, thus making ineffective an approach based on the use of a molecule targeting a single receptor or signal pathway, among many differently deregulated.
The seminal paper of Schulze and colleagues (16), represents a key step for a better understanding of the complex scenario of genetic alterations and related pathways of HCC specifically associated with the etiological background, and consequently, for the development of molecularly-targeted therapies in a tailored approach to patients with HCC. Taking advantage of next generation sequencing (NGS) techniques, by whole exome sequencing (WES), the authors analyzed the whole coding sequence of 243 tumor samples at different stages recapitulating the entire multistep process from dysplastic macronodule to advanced tumor, obtained from several European centers. Such highly innovative and sophisticated technological endeavor led to the identification of (I) specific mutations linked to known risk factors of HCC, such as alcohol and tobacco consumption, and hepatitis B virus (HBV) infection; (II) 11 deregulated signal pathways driven by 161 putative genes; and (III) specific mutational patterns through the different stages of the disease.
The main mutational signatures discovered by the authors, clustered in three major groups, namely CTNNB1, AXIN1, and TP53, each associated with specific environmental risk factors, in particular alcohol toxicity and hepatitis B virus infection. CTNNB1 mutations were strongly associated to alcohol intake. CTNNB1 is the gene encoding for β-catenin, a major component of the WNT/β-catenin signaling, known to be frequently deranged in a variety of chronic inflammatory conditions and in different epithelial cancers. Perturbations of this signaling, derived from both canonical (WNT-related) and non-canonical stimulations, result in the activation of downstream effectors regulating many pro-invasive functions, such as epithelial to mesenchymal transition, cell proliferation and cell reshaping, and enhanced secretion of pro-inflammatory mediators (cytokines and chemokines). Moreover, inappropriate activation of β-catenin leads in the liver, to an expansion of the hepatic progenitor cell compartment along with its phenotype stabilization (17). AXIN1 is another component of the WNT/β-catenin pathway. It binds the cytoplasmic phosphoprotein Dishevelled (downstream of the WNT receptor Frizzled), and, together with other partners (adenomatosis polyposis coli, casein kinase 1, and glycogen synthase kinase 3β), retains β-catenin in the cytoplasm, whereby it can be ubiquitinated, thus preventing its nuclear import and subsequent transcriptional activity (18). Unlike CTNNB1, TP53 gene mutations were significantly linked to HBV infection. TP53 is the gene encoding p53, the tumor suppressor protein most frequently mutated in HCC, which regulates cell cycle and DNA repair mechanisms (for instance, by binding the growth arrest and DNA damage GADD45). The inactivation of p53 protein results in an unbalance of the proliferation/apoptosis ratio, thus leaving unchecked the cell proliferative mechanisms.
Starting from a large amount of genetic mutations [161], the study identifies 11 core pathways consequently aberrantly activated in HCC (Table 1). This finding is of particular importance in an attempt to select patients to the best pharmacological treatment. Notably, the 28% of the genetic alterations detected in HCC patients were potentially suitable of treatment with compounds already approved by the US Food and Drug Administration (FDA), while the percentage increased up to 86% when considering drugs actually under investigation in clinical trials of phase I–III. As many of these mutational signatures can be detected since the early clinical stages, this approach might identify potential therapeutic targets not limited to the advanced HCC, as it is now the case with sorafenib. However, it must be underlined that advanced HCCs display an increased number of mutations amenable of molecular targeting, and thus, the NGS-WES approach might open new opportunities in a clinical stage, which for the time being, is only considered for palliation. In addition to genomic alterations directly responsible for tumor development (‘driver genes’), the NGS-WES analysis pinpoints the presence of genes modulating other critical features of tumor invasiveness, involving the sensitivity to chemotherapeutic drugs, as in NQO1 activation induced by mutations in KEAP1 or NFE2L2, or bursting further signal pathways already deregulated in HCC (RAS/MAPK), as observed with RPS6KA3 mutations. NQO1 encodes a reductase member of the NAD(P)H dehydrogenase family which increases sensitivity to HSP90 inhibitors, a class of potent chemotherapeutic drugs, and thus its modulation may represent a strategy to circumvent drug resistance, a major issue of HCC (19).
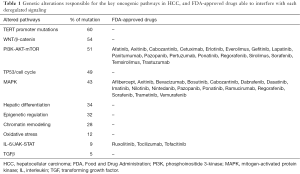
Full table
Furthermore, the study unveils for the first time, gene mutations critically involved in the pathogenetic sequence leading from pre-neoplastic lesions (dysplastic macronodule) to advanced tumor (poor-prognosis HCC) and featuring distinct steps of this process (Figure 1). Whereas mutations of the telomerase reverse transcriptase (TERT) promoter were present in pre-tumoral lesions and in the early stages of HCC, other mutations increased through the progression of the disease (CTNNB1 and TP53); typically, focal mutations of FGF-CCND1 characterized instead the later stages of advanced HCC. Mutations in the TERT promoter represent in particular, a finding of great interest. In fact, TERT promoter mutations were significantly associated with both dysplastic macronodules and early HCC, thus suggesting a pivotal role in the initial phases of malignant transformation. TERT is the catalytic subunit of telomerase, whose function is to restore shortening telomeres, thus preventing replicative senescence, a tumor protective mechanism, and inducing instead a hectic proliferative behavior (20). Reactivation of TERT has been frequently found in various tumors, including HCC, where it was reported in more than 60% of cases (21,22). Interestingly, TERT reactivation has been described also in premalignant lesions of epithelial tumors, such as breast and colon cancer (23), but this is the first time that it is reported in dysplastic foci of liver cirrhosis. Since about 4% of cirrhotic patients will be developing HCC in a year, it is tempting to advance the possibility to identify the dysplastic nodules at major risk of progression to HCC by assessing the TERT promoter mutational signature. This might provide a crucial tool in the generation of new algorithms for the follow-up and treatment of cirrhotic patients. In fact, the finding of TERT promoter reactivation in cirrhotic nodules, especially in diseases related to HBV infection, could orient clinicians to adopt closer surveillance strategies or even lead to very early tailored therapies for these patients.
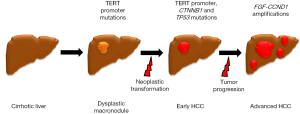
Altogether, the future perspectives of this work are many and of great translational relevance. Within the huge diversity of the HCC world, mutational signatures related to a specific liver background revealed by the NGS-WES analysis, could be used as ‘fingerprints’ to design more appropriately clinical trials, where patients can be better allocated, and to assemble more homogeneous sample cohorts, characterized by single mutational signatures. More in particular, this molecular-based stratification could be a valuable approach to accurately test the efficacy of one or more drugs able to target selectively the deregulated pathway, without confounding factors related to the presence of HCC of different etiologies or harboring different mutational signatures. Moreover, in clinical practice, it would also be possible for the clinician to screen the heterogeneous HCC patient population in distinct subsets to provide them with the most effective personalized treatment and hopefully, to limit the severe side effects caused by the conventional chemotherapy treatments. This approach is predicted to have likely a strong impact on overall and disease free survival of HCC patients in the next years. Unfortunately, the clinical applicability of this approach is actually limited, at least in several European centers. Whilst the costs for the NGS-WES reagents and equipment are continuously decreasing, lowering to about 1,000€ for each sample analysis, there are some major issues that must be contemplated. They include the relative limited availability of instruments even in tertiary care hospitals, and the need of close interactions among different very specialized professional figures, including clinicians, biotechnologists and biostatisticians, which is still difficult to meet. In particular, there is a strong need of skilled biostatisticians, a professional figure actually hard to find, but definitely necessary to allow a fast and thorough analysis of data generated by NGS-WES. Building such a highly specialized team is mandatory for taking full advantage of the wealth of information that this cutting-edge technology may generate for patient care, pursuing the dream of the personalized cure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Guest Editor Haitao Zhao, MD, PhD (Associate Professor, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol 2002;35:S72-8. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006;43:1303-10. [Crossref] [PubMed]
- Wrzesinski SH, Taddei TH, Strazzabosco M. Systemic therapy in hepatocellular carcinoma. Clin Liver Dis 2011;15:423-41. vii-x. [Crossref] [PubMed]
- Lencioni R, Chen XP, Dagher L, et al. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist 2010;15:42-52. [Crossref] [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Gollob JA, Wilhelm S, Carter C, et al. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol 2006;33:392-406. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol 2008;5:194-204. [Crossref] [PubMed]
- Castañeda-Gill JM, Vishwanatha JK. Antiangiogenic mechanisms and factors in breast cancer treatment. J Carcinog 2016;15:1. [Crossref] [PubMed]
- Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015;47:505-11. [Crossref] [PubMed]
- Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol 2012;56:1159-70. [Crossref] [PubMed]
- Li VS, Ng SS, Boersema PJ, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 2012;149:1245-56. [Crossref] [PubMed]
- Gauthier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol Res 2013;43:147-54. [Crossref] [PubMed]
- Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest 2004;113:160-8. [Crossref] [PubMed]
- Nakayama J, Tahara H, Tahara E, et al. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet 1998;18:65-8. [Crossref] [PubMed]
- Nault JC, Zucman-Rossi J. TERT promoter mutations in primary liver tumors. Clin Res Hepatol Gastroenterol 2016;40:9-14. [Crossref] [PubMed]
- Kolquist KA, Ellisen LW, Counter CM, et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet 1998;19:182-6. [Crossref] [PubMed]


