
A lovely leap toward the development of breast cancer therapy with long non-coding RNAs
Breast cancer is among the most popular malignancies by affecting over 10% of women in Western countries (1). It is a highly heterogeneous cancer with various clinical and pathological phenotypes. Due to its popularity and significant threat to the patients’ life, numerous efforts have been conducted for this malignancy in order to establish classification and staging, make early and accurate diagnosis and to develop effective therapeutic regimens (1). The classification of breast cancers using a combination of the three histological markers, the estrogen receptor (ER), progesterone receptor (PR) and the epidermal growth factor receptor 2 (HER2), is successful, predicting in some part prognosis of the harboring patients and helping to choose an effective treatment (1); ER(+), PR(+) and HER2(−) cancers are associated with fair prognosis and good response to anti-estrogen therapy, while ER(−), PR(−), and HER(−) tumors [triple negative breast cancer (TNBC)] are mostly resistant to anti-estrogens and have a poorer prognosis (2). As normal breast tissues are highly responsive to estrogens for their growth and maintenance of their functions, the breast cancers especially those with positive ER expression are also highly dependent on these sex steroids for proliferation (3). The ER that mediates the biologic actions of estrogens is a member of the steroid hormone receptor (SR) family, which also composes of the glucocorticoid, mineralocorticoid, androgen receptor (GR, MR, AR) and PR (4). These receptors act as ligand-dependent transcription factors. Upon binding to their ligand through the C-terminally located ligand-binding domain (LBD), they translocate into the nucleus, and bind, via their DNA-binding domain (DBD), specific DNA recognition sequences called hormone response elements (HREs) located in the promoter region of responsive genes. DNA-associated SRs then regulate the transcriptional activity of these genes positively or negatively via their two transactivation domains located respectively in their N-terminal domain (NTD) and LBD (4). Through ER, estrogens modulate expression of the many molecules involved in survival, proliferation, metastasis or migration of breast cancer cells (3). These actions of estrogen/ER are also influenced by other SRs, such as PR, GR and AR, directly (forming a protein complex with ER) or indirectly (modulating the expression of the genes that affect the activity of ER target proteins) (5).
Recent development and use of the cap analysis of gene expression (CAGE) and high throughput sequencing with next generation sequencers revealed that over half of the human genome expresses RNAs (6). Most of these abundant RNAs are non-protein coding, expressed from the gene area outside the protein-coding sequences, such as intergenic and intronic regions (7). These non-coding (nc) RNAs are empirically categorized into 2 groups, short and long forms, depending on their length of oligonucleotides (7). Representatives of the short ncRNAs are transfer RNAs and micro (mi) RNAs, whereas those for the long ncRNAs (lncRNAs) are ribosomal RNAs. Many of these newly identified ncRNAs are functional even not expressing proteins (6). For example, transfer RNAs are essential for the translation of mRNAs by delivering amino acids to ribosomes, whereas miRNAs spanning ~20–25 bases influence diverse human activities by suppressing mRNA translation or facilitating its degradation (8). Although the physiologic importance of the lncRNAs are still largely unknown, they may also have important biologic actions, as observed in some lncRNAs, such as the X inactive specific transcript (XIST) for inactivation of one of the two X chromosomes existing in male cells and the HOX transcript antisense RNA (HOTAIR) for gene-silencing at the HOX locus in embryo and subsequent segmental body development (8). The growth arrest-specific 5 (Gas5) is one such lncRNA. It is a ~600 bs single stranded RNA having a poly-A tail at its 3’ end. Gas5 was originally identified as the RNA accumulated in growth-arrested cells (9). Its encoding gene, GAS5, has 12 exons in human and is one of the 5’-terminal oligopyrimidine (5’TOP) class genes characterized by the upstream oligopyrimidine tract sequence, which allows Gas5 through inhibition of its degradation to accumulate in nutrient-deficient and growth-arrested cells (9). Biologic actions of Gas5 are not well elucidated so far, but it has multiple activities, such as induction/inhibition of apoptosis, cell cycle arrest at the G0/G1 phase and modulation of protein translation with several proposed mechanisms (10). Gas5 is expressed in various immune cells, and its cellular levels are differentially regulated in some autoimmune and inflammatory disorders, indicating its potential regulatory roles in the immune activity and implication to associated diseases (11). Gas5 also has a binding site for the miRNA miR-21 at its middle portion and functions as a molecular sponge for this miRNA (12). miR-21 is an oncogene affecting mRNAs of a number of genes involved in cell growth, proliferation and apoptosis (12), thus Gas5 may influence the activity of cancer cells through this miRNA. Indeed, Gas5 expression is altered in various cancer tissues, including gastric, liver, pancreatic, colon and uterine cervical cancers, and its levels are positively correlated with prognosis of some of these neoplasms including breast cancer (10). In addition to these activities, Gas5 acts as a potent transcriptional repressor for several SRs, which employ 3-keto steroids as their ligands, such as GR, MR, AR and PR (13). Gas5 has at its 3’ portion the sequence forming the double-stranded hairpin structure and mimicking DNA HREs [thus its was named as “hormone response element mimic” (HREM)], which competes with true DNA HREs for binding to the DBD of these receptors and avoids their access to the DNA elements (13,14) (Figure 1).
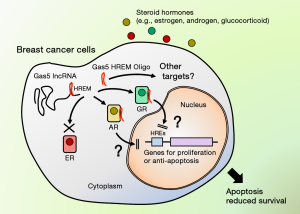
Since Gas5 has a pro-apoptotic activity and its tissue levels are correlated with prognosis of breast cancer patients, Pickard and Williams examined a potential importance of the Gas5 HREM as a therapeutic compound for this cancer in their manuscript appeared in the March issue of Oncotarget (15). They demonstrated that the Gas5 HREM oligonucleotide with 23 bs length preserving the original hairpin structure potently induces apoptosis both in ER-positive, estrogen-sensitive MCF-7 and in ER-negative, TNBC-representing MDA-MB-231 cells (15) (Figure 1). Importantly, this effect of Gas5 HREM oligonucleotide is dependent on the presence of the guanine at position 549 (G549), which is critical for the interaction of Gas5 with SRs (14). Gas5 HREM oligonucleotide also restores the cell death of MCF7 cells, which is attenuated following the reduction of endogenous Gas5 with its siRNA. The authors further employed several chemically modified Gas5 HREM oligonucleotides with an extended half-life, and found that its phosphorothioate form, called as “RNA(PT)-HREM”, demonstrates a strong pro-apoptotic activity and markedly blocks the colony formation of MCF7 cells (15). Because there is a major need to develop new targeted therapies for breast cancers especially the estrogen-resistant forms like TNBC (1), their attempt to use the short oligonucleotides encoding HREM of the Gas5 lncRNA is potentially important for the development of future therapy against this malignancy. Indeed, other researchers developed short chemically modified oligonucleotides called “antagomirs” to block the activity of miRNAs, and some of them are now under clinical trials for the treatment of hepatitis C infection and hepatocellular carcinoma (16). Since lncRNAs are abundant and many of them may have regulatory actions on cell growth, proliferation, differentiation and/or apoptosis (17), they are definitely promising targets for future anti-cancer treatment. Their potential roles in oncogenesis and usefulness as therapeutic targets are suggested by the fact that cancer-associated gene alterations are significantly accumulated in several lncRNAs (18). It has also been shown recently that various cancers demonstrate hyper-activation of the super-enhancers for aberrant expression of oncogenes (19). Enhancers express bidirectional lncRNAs from both their ends called enhancer RNAs (eRNAs), which are essential for the transcriptional regulatory activity of these sequences (20). Thus, eRNAs may also be a promising target for anti-cancer treatment.
Revealing the mechanism(s) underlying the pro-apoptotic actions of the Gas5 HREM oligonucleotide in breast cancer cells is challenging. Since the nucleotide change known to inactivate the ability of Gas5 HREM to bind SRs also abolishes its pro-apoptotic action (14,15), it is highly likely that the effect of the Gas5 HREM oligonucleotide is mediated by some SRs, although it is still possible that the Gas5 HREM interacts with yet unknown molecule(s) through which it exerts the pro-apoptotic effect. While promising based on the pathophysiology of breast cancers, ER is not a target of the Gas5 HREM, as Gas5 cannot bind DBD of this receptor in contrast to the other SRs (14) (Figure 1). This is also consistent with the authors’ results that Gas5 induces apoptosis in the ER-negative, estrogen-resistant cell line (15). The authors exclude PR as a target for Gas5, because PR is not expressed in MDA-MB-231, a model cell line for TNBC in which Gas5 HREM still demonstrates the pro-apoptotic effect (15). Furthermore, MR is not a candidate receptor for Gas5 either, because its expression is highly limited to the organs handling electrolyte metabolism, such as kidney and colon, and breast tissues are not likely to express this receptor. Although the authors did not extend the discussion further, AR might be a candidate receptor responsible for the pro-apoptotic action of the Gas5 HREM oligonucleotide. Some breast cancers, including TNBC, express AR and show androgen-dependent growth in an estrogen-independent manner (21). Several clinical trials also demonstrated that anti-androgens have a treatment benefit to AR-positive TNBC (22). Thus, it is possible that Gas5 HREM suppresses AR-induced proliferation of the breast cancer cells and subsequently induces apoptosis in these cells. GR is another candidate for mediating the pro-apoptotic effect of the Gas5 HREM oligonucleotide. GR ligand glucocorticoid is a well-known inducer of apoptosis in lymphoid cells, but this steroid also acts as an inhibitor of apoptosis in several epithelial cells by inducing the cellular inhibitor of apoptosis 2 (cIAP2) or the baculovirus IAP repeat-containing protein 3 (BIRC3) (23). The cIAP2 gene has a tandem glucocorticoid response elements in its promoter region and glucocorticoid-induced cIAP2 suppresses apoptosis by binding cell death proteases, such as caspase 3, 7 and 9, and by inhibiting their activities (13). In fact, Gas5 was shown to inhibit the apoptosis induced with anti-Fas ligand antibody/interferon γ treatment by stimulating the expression of cIAP2 in HeLa cells (13). Glucocorticoid/GR is also a strong inducer of the breast tumor kinase (Brk) or the protein tyrosine kinase 6, whose expression is elevated in advanced breast cancers and confers their aggressive phenotype (24). This action of glucocorticoid is supported by the fact that glucocorticoid use is associated with poor response to chemotherapy in TNBC (25). Since GR is frequently expressed in both ER-positive and triple negative breast cancers (25), it is possible that Gas5 HREM oligonucleotide inhibits glucocorticoid-dependent induction of Brk, and subsequently suppresses proliferation of breast cancer cells and/or induces their apoptosis. These possibilities should be tested in future by treating breast cancer cells with Gas5 HREM oligonucleotide in the presence or absence of these receptor ligands and by evaluating apoptotic changes.
Finally, the authors have progressed one step forward to breast cancer treatment focusing on lncRNAs, which definitely encourages other basic and clinical researchers to go into this new research area of anti-cancer therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Long Chen (Department of PET-CT center at the Yunnan Tumor Hospital, The Third Affiliated Hospital of Kunming Medical University, Department of Biochemistry and Molecular Biology of Kunming Medical University, Kunming, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spitale A, Mazzola P, Soldini D, et al. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol 2009;20:628-35. [Crossref] [PubMed]
- Dai X, Xiang L, Li T, et al. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J Cancer 2016;7:1281-94. [Crossref] [PubMed]
- Lipovka Y, Konhilas JP. The complex nature of oestrogen signalling in breast cancer: enemy or ally? Biosci Rep 2016;36. [PubMed]
- Kino T. Glucocorticoid receptor. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al., editors. Endotext. South Dartmouth MA: MDText.com, Inc.; 2000.
- Bagamasbad P, Denver RJ. Mechanisms and significance of nuclear receptor auto- and cross-regulation. Gen Comp Endocrinol 2011;170:3-17. [Crossref] [PubMed]
- Mattick JS. RNA regulation: a new genetics? Nat Rev Genet 2004;5:316-23. [Crossref] [PubMed]
- Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature 2012;489:101-8. [Crossref] [PubMed]
- Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012;148:1172-87. [Crossref] [PubMed]
- Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988;54:787-93. [Crossref] [PubMed]
- Pickard MR, Williams GT. Molecular and Cellular Mechanisms of Action of Tumour Suppressor GAS5 LncRNA. Genes (Basel) 2015;6:484-99. [Crossref] [PubMed]
- Mayama T, Marr AK, Kino T. Differential Expression of Glucocorticoid Receptor Noncoding RNA Repressor Gas5 in Autoimmune and Inflammatory Diseases. Horm Metab Res 2016; [Epub ahead of print]. [PubMed]
- Zhang Z, Zhu Z, Watabe K, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ 2013;20:1558-68. [Crossref] [PubMed]
- Kino T, Hurt DE, Ichijo T, et al. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 2010;3:ra8. [Crossref] [PubMed]
- Hudson WH, Pickard MR, de Vera IM, et al. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat Commun 2014;5:5395. [Crossref] [PubMed]
- Pickard MR, Williams GT. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget 2016;7:10104-16. [PubMed]
- Shibata C, Otsuka M, Kishikawa T, et al. Current status of miRNA-targeting therapeutics and preclinical studies against gastroenterological carcinoma. Mol Cell Ther 2013;1:5. [Crossref] [PubMed]
- Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell 2013;154:26-46. [Crossref] [PubMed]
- Nik-Zainal S, Davies H, Staaf J, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 2016;534:47-54. [Crossref] [PubMed]
- Hnisz D, Abraham BJ, Lee TI, et al. Super-enhancers in the control of cell identity and disease. Cell 2013;155:934-47. [Crossref] [PubMed]
- Shlyueva D. Stampfel G1, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet 2014;15:272-86. [Crossref] [PubMed]
- Doane AS, Danso M, Lal P, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006;25:3994-4008. [Crossref] [PubMed]
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Wen LP, Madani K, Fahrni JA, et al. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol 1997;273:L921-9. [PubMed]
- Regan Anderson TM, Ma SH, Raj GV, et al. Breast Tumor Kinase (Brk/PTK6) Is Induced by HIF, Glucocorticoid Receptor, and PELP1-Mediated Stress Signaling in Triple-Negative Breast Cancer. Cancer Res 2016;76:1653-63. [Crossref] [PubMed]
- Chen Z, Lan X, Wu D, et al. Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer. Nat Commun 2015;6:8323. [Crossref] [PubMed]


