
Extracellular vesicles in cancer: current status and challenges
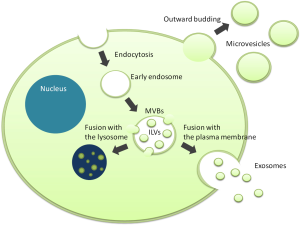
Small membranous vesicles released from the cell surface, which can be frequently observed by electron microscopy, were considered as just an artifact for a long time (Figure 1). The hypothesis that these vesicles, now called extracellular vesicles (EVs), are not mere artifacts but important and primitive cell-cell communication tools, was proposed for the first time in 1984 (1). An Increasing number of studies have demonstrated that EVs contain a variety of biomolecules such as proteins, mRNAs and microRNAs, and that their profiles reflect the state of their donor cells. These cargos can be maintained in a remarkably stable state within biofluids including plasma, urine, saliva, breast milk, and culture media because EVs are composed of a lipid bilayer. Through the horizontal transfer of the cargos, EVs modulate various biological processes in both physiological and pathological conditions. In particular, cancer cells actively secrete and utilize EVs to educate stromal cells in the tumor microenvironment and to arrange the metastatic niche at distant sites for their prosperity. Understanding these roles of EVs has given novel insights into cancer research and encouraged further studies on EVs as potential non-invasive biomarkers and therapeutic targets for cancer. “Liquid biopsy” targeting circulating EVs is now a subject of great interest in cancer diagnosis.
With regard to liquid biopsy, urine can be collected easily, non-invasively and in large volumes compared with the other body fluids. However, EVs isolated from blood have been the focus of EV research so far and studies on urinary EVs have not flourished yet. The recent review article published in European Urology by Junker and colleagues intelligibly organizes the eligible articles regarding urinary EVs in genitourinary tumors (2). To date, 16 studies have examined urinary EVs as biomarkers for bladder, kidney, and prostate cancer and the authors of these studies suggest the great potential of urinary EVs as novel non-invasive biomarkers for these malignancies. However, they also raise several issues in this developing research field.
First, they refer to the current situation that there has been no standardized method for the isolation, confirmation, and quantification of EVs yet. The golden standard method for the isolation of EVs is ultracentrifugation or ultracentrifugation plus filtration, both of which provide fairly pure EVs. In addition, other isolation methods such as sucrose gradient density ultracentrifugation, magnetic beads coated with an EV-specific antibody, and commercially available extraction kits. Methods for the confirmation and quantification of EVs are also varied among studies, for instance, using NanoSight (Malvern Instruments, Malvern, UK) to estimate the size and number of EVs, electron microscopy to confirm their morphological characteristics, and western blot to detect specific markers of EVs (CD9, CD63, and CD81). Furthermore, one of the greatest problems is that a common and reliable internal control for EV-content is not available between independent studies. Therefore, different studies may draw different results even when they examine the same malignancy. Although a consensus has not yet been achieved, the International Society for Extracellular Vesicles (ISEV) is now in a series of enthusiastic discussions regarding the standardization of methods for the isolation and analysis of EVs (3,4).
Second, EVs from different biofluids of the same patient may contain different biomolecules. It is reasonable that the intercellular communication via EVs at one site should be different from that at another site. In their review, the authors refer to a study by Armstrong et al. that compared microRNA profiles of tumor tissue, plasma EVs, urinary EVs, and WBCs from patients with bladder cancer (5), and encourage large-scale profiling studies on EV-contents across biospecimens to discover true and reliable biomarkers. The NanoString nCounter Vantage assay, which Armstrong et al. introduced in their study, seems a fine tool to accelerate these profiling studies.
Third, there is great interest as to how exactly EV-contents are sorted into EVs; are EVs randomly selected packages of molecules or specific molecular groups with the same ultimate goal to manipulate the recipient cells? Accumulating studies on the function of EVs and EV-contents have partially deciphered the contributing factors or unique packaging of specific molecules within EV-contents to the phenotypic alteration of the recipient cells (6,7). However, until now, the majority of EV-research is aimed at biomarker discovery. To use EV-contents as biomarkers, we first need to elucidate their function in each fluid and validate the precise mechanisms by which the cargos are sorted into EVs and released into the biological environment.
The EVs and their contents are now the hot topic in cancer research. Liquid biopsy for EVs is a promising non-invasive method for cancer diagnosis and monitoring in the near future. I hope that the increasing number of studies on EVs will provide us with exciting knowledge and renewed focus to fight cancer.
Acknowledgments
Funding: This work is supported by JSPS KAKENHI (grant number 26870243).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang (Department of Urology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.14). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bastida E, Ordinas A, Escolar G, et al. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood 1984;64:177-84. [PubMed]
- Junker K, Heinzelmann J, Beckham C, et al. Extracellular Vesicles and Their Role in Urologic Malignancies. Eur Urol 2016;70:323-31. [Crossref] [PubMed]
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2. [PubMed]
- Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 2015;4:27031. [Crossref] [PubMed]
- Armstrong DA, Green BB, Seigne JD, et al. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer 2015;14:194. [Crossref] [PubMed]
- Yamada N, Tsujimura N, Kumazaki M, et al. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta 2014;1839:1256-72.
- Yamada N, Kuranaga Y, Kumazaki M, et al. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget 2016;7:27033-43. [PubMed]


