
Chemo-radiotherapy free conditioning regimen: immunotoxin at its magical best
In the June, 2016 issue of Nature Biotechnology, Palchaudhury and colleagues report a novel immunotoxin-based preparative regimen that efficiently conditions immunocompetent mice for autologous hematopoietic stem cell transplantation (HSCT) (1). In comparison with total body irradiation (TBI), there was equivalent endogenous HSC depletion with similar donor chimerism, avoided neutropenia and anemia, enabled rapid immune reconstitution, better preservation of bone marrow architecture and had minimal overall toxicity.
Professor E. Donnall Thomas [1920–2012] got the Nobel Prize for Medicine in the year 1990 for his pioneering work in the field of bone marrow transplantation (BMT), now called allogeneic haematopoietic stem cell transplantation (HSCT). Currently, allogeneic HSCT is being done for the treatment of hematological malignancies (leukemias, lymphomas, myelodysplastic syndrome etc.) and non-haematological conditions e.g., severe aplastic anemia, haemoglobinopathies and immunodeficiency and childhood congenital metabolic disorders etc. Over past four decades procedure has been well established with study of long term effects. This procedure involves conditioning of recipient by high-dose chemotherapy with or without radiotherapy (preparative regimen) followed by stem cell infusion obtained from an HLA-identical donor. This results in engraftment of donor hematopoietic cells following ablation of the recipient's bone marrow (myeloablation) and immune system (immune-ablation). The haematological malignancy is eradicated both by myeloablation and more importantly by the allo-immune reaction (graft-versus-leukaemia or GVL effect) of the engrafted donor cells against residual cells in the recipient. This procedure is not easy to control every time and can lead to conditioning regimen-related organ toxicity (RRT) and graft-versus-host disease (GVHD) where non-haemopoietic cells (e.g., gut, skin, liver, and lung) are targeted. The later remain the major cause of morbidity and mortality. Following success of transplant in a case of Wiskott–Aldrich syndrome in 1978, it was hypothesized that HSCT can potentially cure inherited hematologic disorders. From gene therapy point of view, allogeneic HSCT as a procedure replaces mutant hematopoietic stem cells (HSCs) with functional HSCs from a healthy donor resulting in normalization of the hematopoietic system. Thus, allogeneic HSCT is potentially curative option for most patients with malignant and non-malignant hematological disorders, transplant related morbidity and mortality remains a major deterrent. In addition, a number of long-term complications (an effect of conditioning regimen) e.g., infertility and gonadal failure, growth disturbance, chronic GVHD, and a risk for secondary malignancies are other important limitations (2).
In search for better conditioning regimen, reduced-intensity-conditioning (RIC) regimen was developed in early 2000. This uses lower doses of chemotherapy and radiation than the standard myeloablative conditioning (MAC) but more of immune-ablation with use of drugs e.g., ATG, fludarabine. This novel approach results in less or limited myeloablation but sufficient lymphoablation, which is enough to achieve donor chimerism (mostly mixed in initial days). This approach was associated with reduction in early regimen-related toxicities and is considered as an option for patients with hematologic malignancies who are not eligible for standard allograft either because of age or major co-morbidities. There was further support for this approach when it was observed that mixed chimerism remained stable in patients transplanted for hemoglobinopathies (3). For such patients, mixed chimerism even with a low fraction of donor marrow progenitors (20–30%), have significant enrichment of erythrocyte over leukocyte chimerism which improves their hematological parameters (4). In addition, the presence of residual host cells during the mixed chimerism state also offer an advantage of reducing the incidence and severity of GVHD due to central immune tolerance (5).
Three main objectives of conditioning prior to allogeneic HSCT are (I) to create space for the donor HSCs to home in; (II) to provide immunosuppression for favorable engraftment; and (III) eradication of the residual disease. Conventional MAC regimens (including TBI and cytotoxic agents) not only play a role in the creation of space for donor HSC but also act as immune suppressants to enhance the engraftment further. In immunodeficient mice, an omission of conditioning resulted in very low donor HSC chimerism as the endogenous HSC niches were too few for the purified donor HSCs to access (6). The low-level mixed donor chimerism though sufficient to restore immune function as evidenced in clinical transplantation, at times result in rejection of graft (4,7). So, the existing host stem cells must be eradicated by the conditioning regimen in order to create space for the donor HSCs to access these niches to obtain the necessary support for proliferation and differentiation and result in favorable engraftment as proposed by several studies (8,9).
The principle behind allogeneic HSCT for non-malignant disorder is to create a favorable niche for the donor HSCs to engraft as well as to prevent rejection of donor HSCs achieving a chimerism sufficient enough to produce a functional cure of the underlying disease. HSCT using genetically corrected autologous stem cells after its initial success (10) is emerging as a valid alternative to allogeneic HSCT and will avoid complications like GVHD. But the conditioning regimen may still have to eradicate the defective endogenous HSCs from the niche for these genetically corrected autologous HSCs to home in with utmost safety to the patient.
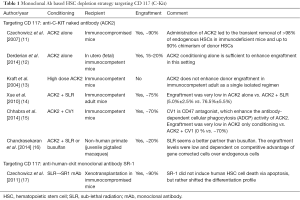
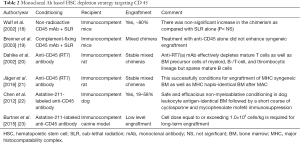
Modern approaches to anticancer drug development are based on Paul Ehrlich’s concept of ‘Magic Bullet’ in the early 1900s. Since then, polyclonal and later monoclonal antibodies have been developed for targeting drugs, toxins etc. with the aim of developing targeted therapies for cancer. These have the advantage of being highly cytotoxic to the targets and easy to be manipulated by genetic engineering methods. This concept has been evaluated to specifically target the HSCs in the BM niche and spare the non-hematopoietic cells, thereby decreasing the morbidity as well as mortality due to off-target toxicity. The targets that have been evaluated in mice are monoclonal antibodies against HSC antigens like CD117 (C-Kit) and CD45 as summarized in Tables 1,2.
Full table
Full table
C-Kit interacts with its ligand, stem cell factor (SCF), which is a key signaling pathway involved in homing, adhesion, maintenance, and survival of HSCs in the hematopoietic niche (24,25). HSCs from all stages of development express the same levels of the C-Kit receptor or CD117 on the cell surface and as HSC differentiates to mature progeny expression of C-Kit is down regulated (24,25). Therefore, an antibody against the C-Kit receptor will deplete endogenous HSCs selectively than the mature progenitors. C-Kit signaling inhibition seems a feasible approach in immunodeficient mice (11) and to some extent in fetal immunocompetent mice (in-utero transplant) (12). However, in immunocompetent adult mice, it failed when used alone (13) and required additional radiation or chemotherapy or CD47 blockade (which enhance antibody-dependent cellular phagocytosis) to achieve desirable chimerism (Table 1) (14,15).
It was observed that there was a lower hematological relapse with a higher dose of TBI in conditioning (16 vs. 12 Gy) but at a cost of higher mortality because of higher radiation dose to important organs like liver and lungs (26). So, further improvement in allogeneic HSCT may still be possible if the radiation dose could still be increased at the level of bone marrow without any additional toxicity.
CD45 is a tyrosine phosphatase stably expressed on virtually all hematopoietic cells and absent in non-hematopoietic tissues and was tagged with high energy emitting radioisotope in one study (27). Subjects with high-risk hematological malignancies were focus of studies using anti-CD45 antibody with (22,23) or without (18-21) radioisotope in addition to DNA damaging agents (Table 2). This approach was associated with modest success in mice (18-21) and dogs (22,23).
In the present study, Palchaudhuri et al. (1) have taken one step further; an anti-CD45 antibody was coupled with saporin (SAP), which is a ricin-family toxin without a general cell entry domain and halts protein synthesis only upon receptor-mediated internalization into the cytoplasm. Among several cell-surface markers tested (like CD 45, CD49D, CD84, CD90, CD133, CD135 and CD184), only the immunotoxin targeting CD45 was capable of depleting endogenous HSCs. This was probably related to the amount of expression and the extent of internalization. The internalization frequency of the present immunotoxin was 12% over a 24-hour period, which was sufficient for potent HSC depletion in immunocompetent mice and also enables sufficient and durable donor-cell engraftment (75–90% at 4 months post-HSCT) and was comparable to 5 Gy TBI and a lot better than ACK2 alone (<3%). ACK2, failed as an isolated agent in this immunocompetent mice setting even when an immunotoxin was coupled (ACK2-SAP) (13). Apart from this, there was also significant synergy between 3 mg/kg CD45-SAP and 5 Gy TBI with donor chimerism increasing up to 90%.
In contrast to 5 Gy TBI, CD45-SAP also preserved the bone marrow architecture, as it was less detrimental to bone marrow cellularity, hematopoietic progenitors, and bone marrow microenvironment. It also preserves innate immunity, avoids anemia and facilitates rapid B- and T-lymphocyte recovery as compared to TBI. They also demonstrated that CD45-SAP induces rapid and transient myeloid and lymphoid cell depletion and a stronger, long lasting depletion of HSCs in mice. When used alone as a conditioning agent for HSCT in immunocompetent mice, the treatment is nearly as effective as TBI at establishing long-term stable donor cell chimerism but with substantially less toxicity, as shown by the faster recovery of myeloid, B cells and T cells in the blood with better preservation of bone marrow and thymus architecture.
And finally in a sickle cell disease (SCD) mouse model this innovative conditioning at a higher dose of 4–6 mg/kg was able to achieve >90% donor myeloid chimerism at 4 months with complete normalization of RBC parameters as well as non-sickled morphology in peripheral smear and normal haemoglobin protein by native-PAGE analysis. More importantly, the spleen size also returned to normal size as compared to wild-type, the information about the normalization of splenic haematopoiesis was not detailed in the present study (1). Spleen in mice serves as the normal site of hematopoiesis like bone marrow in humans. Spleen in SCD mouse model is enlarged because of high erythropoietic rate due to hemolysis because of size-constrained limits on bone marrow hematopoiesis (4). Therefore, murine splenic hematopoiesis likely serves as a model of human bone marrow hematopoiesis (4) and in the present study has possibly improved as evidenced by their corresponding reduction in splenomegaly. But despite all the above inferences, the question still remains about the minimum level of modification required from conditioning to derive curative outcome in SCD model.
This innovative immunotoxin-based conditioning regimen of Palchaudhuri et al. (1) will require further research to determine how far these results in mice can be extrapolated in humans. A potential limitation of this method of conditioning is the failure to deplete endogenous HSCs completely to establish a complete donor cell chimerism. But in the SCD mouse model, it was observed that increasing the dose from to 3 to 4–6 mg/kg is feasible and possibly achieves a better chimerism status in the higher dose subset (75–90% in 3 mg/kg vs. >90% in 4–6 mg/kg). Thus, it seems there is still a lot of scope to improve upon the current results. Again, mice and humans differ in many aspects including size, environmental conditions (e.g., controlled environment conditions in a lab for mice) and lifespan, which impose different selective proliferative pressures on HSCs from each species (28). As a result, the functional aspect of human HSCs is different with respect to self-renewal or quiescence as compared to mouse HSCs. For instance, competitive transplantation studies in mice have estimated that murine HSCs divide every 2.5 weeks, which is significantly faster than human HSCs (every 40–45 weeks) (28). Again, the number of red blood cells (RBCs) produced by mice in a 2-year lifespan is comparable to the number of RBCs produced daily by a healthy adult individual (28). So, these results need to be interpreted with caution and possibly needs replication in non-human primate models. Team led by Prof. David T. Scadden must be congratulated for this outstanding effort in this direction. These results came at a time when lot of development is happening in the era of gene therapy and gene editing in haemoglobinopathies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palchaudhuri R, Saez B, Hoggatt J, et al. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol 2016;34:738-45. [Crossref] [PubMed]
- Locatelli F, Giorgiani G, Pession A, et al. Late effects in children after bone marrow transplantation: a review. Haematologica 1993;78:319-28. [PubMed]
- Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant 2001;7:665-73. [Crossref] [PubMed]
- Kean LS, Manci EA, Perry J, et al. Chimerism and cure: hematologic and pathologic correction of murine sickle cell disease. Blood 2003;102:4582-93. [Crossref] [PubMed]
- Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation 1999;68:459-67. [Crossref] [PubMed]
- Bhattacharya D, Rossi DJ, Bryder D, et al. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med 2006;203:73-85. [Crossref] [PubMed]
- Cavazzana-Calvo M, Carlier F, Le Deist F, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood 2007;109:4575-81. [Crossref] [PubMed]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978;4:7-25. [PubMed]
- Micklem HS, Clarke CM, Evans EP, et al. Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation 1968;6:299-302. [Crossref] [PubMed]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002;346:1185-93. [Crossref] [PubMed]
- Czechowicz A, Kraft D, Weissman IL, et al. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 2007;318:1296-9. [Crossref] [PubMed]
- Derderian SC, Togarrati PP, King C, et al. In utero depletion of fetal hematopoietic stem cells improves engraftment after neonatal transplantation in mice. Blood 2014;124:973-80. [Crossref] [PubMed]
- Kraft DL, Weissman IL. Effect and kinetics of depleting ACK--2 Anti C- Kit monoclonal antibody on hematopoeisis and hematopoetic progenitors and ability to condition for bone marrow transplantation. ASH Annual Meeting Abstracts 2004;104:4963.
- Xue X, Pech NK, Shelley WC, et al. Antibody targeting KIT as pretransplantation conditioning in immunocompetent mice. Blood 2010;116:5419-22. [Crossref] [PubMed]
- Chhabra A, Ring AM, Weiskopf K, et al. Successful Engraftment of Hematopoietic Stem Cells into Immunocompetent Recipients Using Only Anti-CD117 Antibody and CD47 Blockade As Conditioning. Blood 2014;124:2410.
- Chandrasekaran D, Nakamoto B, Watts KL, et al. Modeling promising nonmyeloablative conditioning regimens in nonhuman primates. Hum Gene Ther 2014;25:1013-22. [Crossref] [PubMed]
- Czechowicz A, Bhardwaj R, Pang W, et al. Targeted Clearance of Human Hematopoietic Stem Cell Niches Via Inhibition of SCF Signaling Using Monoclonal Antibody SR-1. Biol Blood Marrow Transplant 2011;17:S187-8. [Crossref]
- Wulf GG, Luo KL, Goodell MA, et al. Anti-CD45-mediated cytoreduction to facilitate allogeneic stem cell transplantation. Blood 2003;101:2434-9. [Crossref] [PubMed]
- Brenner MK, Wulf GG, Rill DR, et al. Complement-fixing CD45 monoclonal antibodies to facilitate stem cell transplantation in mouse and man. Ann N Y Acad Sci 2003;996:80-8. [Crossref] [PubMed]
- Dahlke MH, Lauth OS, Jäger MD, et al. In vivo depletion of hematopoietic stem cells in the rat by an anti-CD45 (RT7) antibody. Blood 2002;99:3566-72. [Crossref] [PubMed]
- Jäger MD, Vondran FW, Ramackers W, et al. A Depleting Anti-CD45 Monoclonal Antibody as Isolated Conditioning for Bone Marrow Transplantation in the Rat. PLoS One 2016;11:e0154682 [Crossref] [PubMed]
- Chen Y, Kornblit B, Hamlin DK, et al. Durable donor engraftment after radioimmunotherapy using α-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood 2012;119:1130-8. [Crossref] [PubMed]
- Burtner CR, Chandrasekaran D, Santos EB, et al. (211)Astatine-Conjugated Monoclonal CD45 Antibody-Based Nonmyeloablative Conditioning for Stem Cell Gene Therapy. Hum Gene Ther 2015;26:399-406. [Crossref] [PubMed]
- Ogawa M, Matsuzaki Y, Nishikawa S, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med 1991;174:63-71. [Crossref] [PubMed]
- Palmqvist L, Glover CH, Hsu L, et al. Correlation of murine embryonic stem cell gene expression profiles with functional measures of pluripotency. Stem Cells 2005;23:663-80. [Crossref] [PubMed]
- Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood 1990;76:1867-71. [PubMed]
- Press OW, Howell-Clark J, Anderson S, et al. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood 1994;83:1390-7. [PubMed]
- Larochelle A, Dunbar CE. Hematopoietic stem cell gene therapy:assessing the relevance of preclinical models. Semin Hematol 2013;50:101-30. [Crossref] [PubMed]


