
Impact of the PARP1 rs1136410 and rs3219145 polymorphisms on susceptibility and clinicopathologic features of breast cancer in a Chinese population
Introduction
Breast cancer (BC), with nearly 1.7 million incidence and 522,000 deaths (according to 2012 GLOBOCAN statistics), is the most frequently occurring cancer in women. Actually, the BC rates in Asia exceeded the historically high rates in the United States in recent generations (1,2). In China [2011], although BC was only the sixth leading cause of cancer death (mortality was 9.21/105) in females, it was the most common cancer among women overall (incidence was 37.86/105) (3). The etiology of BC, which is thought to be multifactorial, has not been completely elucidated. But it is widely known that genetic factors contribute to an increased or a decreased BC susceptibility, which means an important role of genetic variations to BC risk (4,5).
Poly(ADP-ribose) polymerase-1 (PARP1) is the main part of the PARP family. Activated by DNA breaks, it plays important roles in DNA repair and other cellular processes (6). PARP1 can induce cell survival by repairing DNA, but it is degraded during apoptosis by caspases (7). Its overexpression contributes to the development of various tumors (8,9). In triple-negative BC and other human cancer types, the expression of PARP1 was upregulated (10), and its nuclear expression was linked with chemotherapy response in invasive primary BCs (11). PARP1/2 inhibitors (olaparib, for example) as therapeutic agents are currently used in clinical trials in breast and ovarian cancer (12,13). However, some studies revealed that PARP1 participated in inhibiting malignancy in mice, and the reduction of PARP1 activity in human peripheral blood lymphocytes is connected with various cancers (14,15). The explanation for these contrary findings remains undiscovered, but single nucleotide polymorphisms (SNPs) may play roles in the different functions of PARP1.
To date, there are at least 400 SNPs, including 17 non-synonymous SNPs (nsSNPs) in the PARP1, but functional analyses have only been performed about the rs1136410 and rs3219145 polymorphisms (16). The impact of the PARP1 polymorphisms is currently unclear. Some studies have demonstrated an association of that PARP1 polymorphisms and an increased tumors risk including stomach (17), esophageal (18), cervical (19) and lung (20). In contrast, other studies have reported that PARP1 polymorphisms are associated with reduced risk of malignancy including glioma and non-Hodgkin lymphoma (21,22). In some researches on BC, the PAPR1 polymorphism (rs1136410) increased tumor risk among Saudi and Asian population, but decreased risk of cancer among Caucasians. Therefore, the PAPR1 polymorphisms might play different roles in different ethnic populations and different cancer types (23,24). Because of these uncertain results, and the fact that only a few studies on BC involved Asian populations, we conducted this case-control study to determine the associations between the PARP1 rs1136410 and rs3219145 polymorphisms and BC risk in a Chinese population.
Methods
Study participants
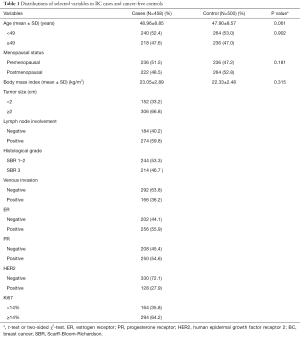
All 458 cases were consecutively recruited from August 2013 to September 2014 from the Second Affiliated Hospital of Xi’an Jiaotong University, while the 500 controls came from volunteers. These 958 participants signed informed consent documents during face-to-face interviews before recruitment and they understood the purpose of the research. All of the patients were sporadic BC by pathologically confirmation. Patients would be excluded if they ever had other types of cancer. For comparison, the control and case groups were frequency-matched based on age (48.96 vs. 47.90, P=0.061), as shown in Table 1. The basic information about the two groups is showed in Table 1. The Institutional Review Board of the Second Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China) approved this research (No. 2015-010) (25-27).
Full table
SNP selection and genotyping
We selected two SNPs (rs1136410 and rs3219145) from the PARP1 gene, which had previously been shown to be associated with various tumors (16-20,23). We used proteinase K digestion and phenol/chloroform extraction to isolate and purify genome DNA from peripheral blood leukocytes, as described previously (4,5), and measured the concentration using spectrophotometry (DU 530 UV/Vis spectrophotometer, Beckman instruments, Fullerton, CA, USA). The following corresponding primers were used for SNPs in this study: for rs1136410, forward primer: 5'-ACGTTGGATGCACCATGATACCTAAGTCGG-3' and, reverse primer: 5'-ACGTTGGATGATGTCCAGCAGGTTGTCAAG-3'; for rs321945, forward primer: 5'-ACGTTGGATGTGTTGCCATCTTAATCTCAG-3' and, reverse primer: 5'-ACGTTGGATGTTGAGTTTTGCCCCTCAGTC-3'. The two SNPs were genotyped by the Sequenom MassARRAY RS1000 (Sequenom, Inc., San Diego, CA, USA) following the manufacturer’s instructions. The data was managed and analyzed by Sequenom Typer 4.0 software.
Statistical analysis
Statistical analyses were conducted with the Student’s t-test for continuous variables and the χ2-test for categorical variables by SPSS PASW Statistics v18.0 (SPSS Inc., Chicago, IL, USA). For controls, the allele frequencies were assessed whether they deviated from the Hardy-Weinberg equilibrium (HWE) using the χ2-test before analysis. We performed unconditional logistic regression to evaluate the associations between the two PARP1 polymorphisms and BC risk were evaluated by calculating odds ratios (ORs) and 95% confidence intervals (CIs), and P values were adjusted for age, menopausal status, and body mass index. The associations between the PARP1 genotypes of the polymorphisms and patients’ clinical characteristics were estimated by the χ2-test and ORs and 95% CIs. Significance was taken when P<0.05, and all statistical tests were two-sided.
Results
Characteristics of the patients and controls
The basic characteristics of the two groups are showed in Table 1. There had no significant differences between the cases and controls for the age (P=0.061), the stratification of age (P=0.902) and the menopausal status (P=0.181). The percentages of patients with tumors <2 and ≥2 cm in size were 33.2% and 66.8%, respectively. About 47% of the patients had Scarff-Bloom-Richardson (SBR) 3 grade cancer. The percentages of patients with lymph node involvement and venous invasion were 59.8% and 36.2%, respectively. In addition, patients with estrogen receptor- (ER-), progesterone receptor- (PR-), human epidermal growth factor receptor 2- (HER2-) and Ki67-positive disease account for 55.9%, 54.6%, 27.9%, and 64.2% of the overall cases, respectively.
Association between the PARP1 polymorphism and the risk of BC
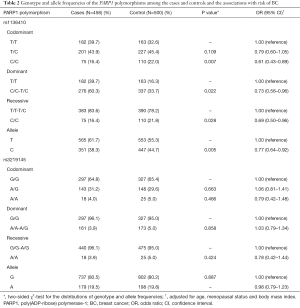
The genotype distributions and alleles of the PARP1 rs1136410 and rs3219145 polymorphisms are presented in Table 2. The two SNPs’ genotype distribution was in HWE in controls tested (the P values were 0.07 and 0.13 for the rs1136410 and rs3219145 polymorphisms, respectively). The frequencies of the PARP1 rs1136410 genotypes were significantly different between the case and control groups (P=0.024). The same trend was also observed between cases and controls under the allele model (P=0.005). Subjects with the C/C genotype had a lower BC risk than those with T/T genotype (P=0.007, OR =0.61, 95% CI: 0.43–0.89) and TT/TC genotypes (P=0.028, OR =0.69, 95% CI: 0.50–0.96). However, we could not find any association between the PARP1 rs3219145 polymorphism and BC risk in any comparison.
Full table
Stratified analysis of the PARP1 rs1136410 polymorphism and risk of BC
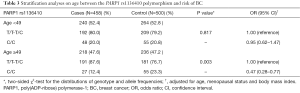
Stratified by age, we investigated the influence of the PARP1 rs1136410 polymorphism on BC risk. As shown in Table 3, the protective effect of the PARP1 rs1136410 C/C genotype was confirmed in older subjects (P=0.003, OR =0.47, 95% CI: 0.28–0.77) rather than younger subjects, which suggested that older individuals could benefit more from carrying the C/C genotype. The same analysis was also performed for the PARP1 rs3219145 polymorphism, but got no significant observations (data not shown).
Full table
Association between the PARP1 rs1136410 polymorphism and clinical parameters of BC patients
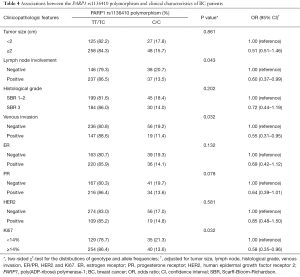
On the basis of the clinicopathologic features of BC patients, we then analyzed the association between the PARP1 polymorphisms and a series of clinicopathologic features including tumor size, lymph node involvement, histological grade, venous invasion, ER/PR, HER2, and Ki67. In patients with positive lymph node involvement, positive venous invasion and Ki67 index ≥14%, the frequencies of CC genotype were 13.5%, 11.4%, and 13.6% versus 86.5%, 88.6% and 86.4% in those with TT/TC genotypes, respectively. No other significant association between the PARP1 polymorphisms and the clinical features was observed, as shown in Table 4.
Full table
Discussion
PARP1 is the main member of the PARP family and participates in DNA repair pathways. It can influence carcinogenesis and tumor biology by inducing cell survival or impacting apoptosis (10). Although the overexpression of PARP1 was confirmed in numerous BC studies, some contrary results have also been reported (8-15). Therefore, the role of PARP1 remains uncertain, and the same is true for PARP1 polymorphisms. There were significantly different of the PARP1 rs1136410 genotypes between the case and control groups. Compared to individuals with the C/C and CC/TC genotypes, those with the C/C genotype had a more decreased BC risk. However, we could not find any relationships between the PARP1 rs3219145 polymorphism and BC susceptibility in any comparison. Interesting, our results were in contrast to those of Alanazi (24), who found in a Saudi population that the PARP1 rs1136410 increased the risk of BC. Recently, a meta-analysis found evidence that the association of the PARP1 polymorphism with risk of cancer was contrary in different populations (23). Our results provide the first evidence that the PARP1 rs1136410 polymorphism was associated with a decreased risk of BC in a Chinese population. V762A (rs1136410) based on T to C transition at codon 762 in exon 17 in PARP1, this transition resulted in the substitution of alanine for valine in the catalytic domain of PARP1 protein and was associated with an altered activity of PARP1. This might be the molecular mechanism of the protective role of PARP1 rs1136410 in BC, but further experiments are needed to verify.
PARP1 expression has been correlated with clinicopathologic characteristics, outcome, and some DNA repair proteins’ expression. In particular, PARP1 expression was positively related to younger premenopausal patients, and those with larger size tumors and higher tumor grade (28), whether PARP1 rs1136410 would have similar associations with clinicopathologic features of BC. In the current study, we found that the frequency of the C/C genotype was significantly lower in patients with lymph node involvement, venous invasion, and Ki67 positivity, suggesting that the variant genotype of this polymorphism may play a protective role during the BC progression. We found no other associations between the PARP1 polymorphisms and the clinical features of BC. Lymph node involvement, venous invasion, and Ki67 are poor prognostic factors. Our results indicated the higher expression of Ki67 and the more lymph node involvement with the lower expression of C/C type, this result was agree with the absence of C/C protective role. However, the molecular mechanism remains to be studied. Fortunately, the variant genotype (C/C) had a protective impact in this population. Although PARP1 inhibitors as therapeutic agents are being used in clinical trials, especially for patients with triple-negative BC, in we did not find any association between PARP1 rs1136410 and ER/PR/HER2. In patients with BRCA1- or BRCA2-mutated HER2-negative advanced BC, the PARP1 inhibitor (olaparib) combined with carboplatin in a randomized phase II trial acquired positive results (29), but in other research patients without BRCA1- or BRCA2-mutation the PARP1 inhibitors were also effective (30). Whether this phenomenon was related to the PARP1 polymorphisms remained unknown, so future studies with a more specific focus on these topics will be useful.
In the subgroup analysis, we also found that the older individuals (age ≥49 years) could benefit more from carrying the CC genotype. As it is known that more DNA lesions occur as individuals’ age, our results suggest that the CC genotype might play a protective role during this process, and the risk of BC development in younger individuals is influenced by other factors.
Our study does have a particular limitation that should be considered. Since our study was a case-control design, there may exist some bias, for example selection bias, which may result from selected participants with a particular genotype. However, the genotype distributions of PARP1 polymorphisms in our control group all in HWE, suggesting that the selection bias would not be a major concern.
Conclusions
In conclusion, our results suggest that the PARP1 rs1136410 reduce the BC risk and delay BC progression rather than the rs3219145 polymorphism in the Chinese population. Although we demonstrated a statistically significant association in our study, it needs to be confirmed by other larger scale studies.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation, China (No. 81471670; 81274136); China Postdoctoral Science Foundation (No. 2015T81037); the International Cooperative Project of Shanxi province, People’s Republic of China (No. 2016KW-008) and the Fundamental Research Funds for the Central Universities, China (No. 2014qngz-04); Natural Science Foundation research project of Shanxi Province, China (No. 2015JM8430).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the Second Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China) approved this research (No. 2015-010) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tao Z, Shi A, Lu C, et al. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys 2015;72:333-8. [Crossref] [PubMed]
- Sung H, Rosenberg PS, Chen WQ, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst 2015;107:djv107 [Crossref] [PubMed]
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2-12. [Crossref] [PubMed]
- Kang H, Dai Z, Ma X, et al. A genetic variant in the promoter of APE1 gene (-656 T>G) is associated with breast cancer risk and progression in a Chinese population. Gene 2013;531:97-100. [Crossref] [PubMed]
- Ma X, Kang H, Dai Z, et al. Impact of the IGFBP3 A-202C polymorphism on susceptibility and clinicopathologic features of breast cancer. Biomed Pharmacother 2015;71:108-11. [Crossref] [PubMed]
- Lockett KL, Hall MC, Xu J, et al. The ADPRT V762A genetic variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer Res 2004;64:6344-8. [Crossref] [PubMed]
- Negri C, Bernardi R, Braghetti A, et al. The effect of the chemotherapeutic drug VP-16 on poly(ADP-ribosylation) in apoptotic HeLa cells. Carcinogenesis 1993;14:2559-64. [Crossref] [PubMed]
- Nosho K, Yamamoto H, Mikami M, et al. Overexpression of poly(ADP-ribose) polymerase-1 (PARP-1) in the early stage of colorectal carcinogenesis. Eur J Cancer 2006;42:2374-81. [Crossref] [PubMed]
- Quiles-Perez R, Muñoz-Gámez JA, Ruiz-Extremera A, et al. Inhibition of poly adenosine diphosphate-ribose polymerase decreases hepatocellular carcinoma growth by modulation of tumor-related gene expression. Hepatology 2010;51:255-66. [Crossref] [PubMed]
- Ossovskaya V, Koo IC, Kaldjian EP, et al. Upregulation of Poly(ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010;1:812-21. [Crossref] [PubMed]
- Zhai L, Li S, Li X, et al. The nuclear expression of Poly(ADP-ribose) polymerase-1 (PARP1) in invasive primary breast tumors is associated with chemotherapy sensitivity. Pathol Res Pract 2015;211:130-7. [Crossref] [PubMed]
- Lee JM, Hays JL, Annunziata CM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst 2014;106:dju089 [Crossref] [PubMed]
- Jamdade VS, Sethi N, Mundhe NA, et al. Therapeutic targets of triple-negative breast cancer: a review. Br J Pharmacol 2015;172:4228-37. [Crossref] [PubMed]
- Rajaee-Behbahani N, Schmezer P, Ramroth H, et al. Reduced poly(ADP-ribosyl)ation in lymphocytes of laryngeal cancer patients: results of a case-control study. Int J Cancer 2002;98:780-4. [Crossref] [PubMed]
- Tong WM, Yang YG, Cao WH, et al. Poly(ADP-ribose) polymerase-1 plays a role in suppressing mammary tumourigenesis in mice. Oncogene 2007;26:3857-67. [Crossref] [PubMed]
- Figueroa JD, Malats N, Real FX, et al. Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet 2007;121:233-42. [Crossref] [PubMed]
- Zhang Q, Li Y, Li X, et al. PARP-1 Val762Ala polymorphism, CagA+ H. pylori infection and risk for gastric cancer in Han Chinese population. Mol Biol Rep 2009;36:1461-7. [Crossref] [PubMed]
- Hao B, Wang H, Zhou K, et al. Identification of genetic variants in base excision repair pathway and their associations with risk of esophageal squamous cell carcinoma. Cancer Res 2004;64:4378-84. [Crossref] [PubMed]
- Roszak A, Lianeri M, Sowińska A, et al. Involvement of PARP-1 Val762Ala polymorphism in the onset of cervical cancer in caucasian women. Mol Diagn Ther 2013;17:239-45. [Crossref] [PubMed]
- Zhang X, Miao X, Liang G, et al. Polymorphisms in DNA base excision repair genes ADPRT and XRCC1 and risk of lung cancer. Cancer Res 2005;65:722-6. [PubMed]
- Liu Y, Scheurer ME, El-Zein R, et al. Association and interactions between DNA repair gene polymorphisms and adult glioma. Cancer Epidemiol Biomarkers Prev 2009;18:204-14. [Crossref] [PubMed]
- Jin XM, Kim HN, Lee IK, et al. PARP-1 Val762Ala polymorphism is associated with reduced risk of non-Hodgkin lymphoma in Korean males. BMC Med Genet 2010;11:38. [Crossref] [PubMed]
- Yu H, Ma H, Yin M, et al. Association between PARP-1 V762A polymorphism and cancer susceptibility: a meta-analysis. Genet Epidemiol 2012;36:56-65. [Crossref] [PubMed]
- Alanazi M, Pathan AA, Abduljaleel Z, et al. Association between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi population. PLoS One 2013;8:e85541 [Crossref] [PubMed]
- Dai ZJ, Liu XH, Kang HF, et al. Genetic Variation in Metastasis-Associated in Colon Cancer-1 and the Risk of Breast Cancer Among the Chinese Han Population: A STROBE-Compliant Observational Study. Medicine (Baltimore) 2016;95:e2801 [Crossref] [PubMed]
- Dai ZJ, Liu XH, Ma YF, et al. Association Between Single Nucleotide Polymorphisms in DNA Polymerase Kappa Gene and Breast Cancer Risk in Chinese Han Population: A STROBE-Compliant Observational Study. Medicine (Baltimore) 2016;95:e2466 [Crossref] [PubMed]
- Wang M, Wang X, Fu SW, et al. Single-nucleotide polymorphisms in PSCA and the risk of breast cancer in a Chinese population. Oncotarget 2016;7:27665-75. [PubMed]
- Green AR, Caracappa D, Benhasouna AA, et al. Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res Treat 2015;149:353-62. [Crossref] [PubMed]
- Schouten PC, Dackus GM, Marchetti S, et al. A phase I followed by a randomized phase II trial of two cycles carboplatin-olaparib followed by olaparib monotherapy versus capecitabine in BRCA1- or BRCA2-mutated HER2-negative advanced breast cancer as first line treatment (REVIVAL): study protocol for a randomized controlled trial. Trials 2016;17:293. [Crossref] [PubMed]
- Sistigu A, Manic G, Obrist F, et al. Trial watch - inhibiting PARP enzymes for anticancer therapy. Mol Cell Oncol 2015;3:e1053594 [Crossref] [PubMed]


