
Cyclin dependent kinase 5—a novel target to enhance the antitumor immune response
Cyclin dependent kinase 5 (Cdk5) is a serine/threonine kinase that has initially been discovered in the neuronal system and is essential for CNS development and function (1). During recent years, important functions of Cdk5 in various tumors have been elucidated and the genetic knockdown of Cdk5 as well as the inhibition of its kinase activity by small molecules has shown potent anti-cancer effects (2-5). Therefore, Cdk5 has emerged as a promising target for anti-cancer therapy.
Dorand et al. now elucidate a novel function of Cdk5 in cancer: their findings highlight a central role of Cdk5 in antitumor immunity (6). Cancer cells often activate immune-inhibitory signals that allow the tumor to escape immune surveillance (7). Cancer immunotherapy by targeting immune checkpoints such as the programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway enables the development of antitumor immune responses and has achieved major therapeutic benefit (8). According to the study by Dorand et al., inhibition of Cdk5 might represent a new option to enhance the antitumor immune response.
By using subcutaneous medulloblastoma (MB) tumor models, Dorand et al. show that, whereas Cdk5 deficient tumors grew normally in immunodeficient mice, in immunocompetent C57BL/6 mice implanted with Cdk5-deficient tumor cells tumor-free survival was strongly increased and tumor size was reduced compared to mice implanted with control tumors. Moreover, Dorand et al. observed an inverse correlation of Cdk5 expression and T cell infiltration in human MB, suggesting that the rejection of Cdk5-deficient tumors was T cell dependent. In fact, Dorand et al. could trace the rejection of Cdk5-deficient tumors back to CD4+ T cells as the depletion of CD4+ T-cells or CD4+ and CD8+ T-cells together abrogated the reduced tumor incidence and the growth of Cdk5-deficient tumors whereas the depletion of only CD8+ T cells had no effect. Moreover, Cdk5-deficient tumors grew normally in mice deficient for major histocompatibility complex II (MHC-II) which is recognized by the T cell CD4 co-receptor. Importantly, injection of WT-tumor cells into mice that had rejected Cdk5-deficient tumors before did not result in tumor establishment, meaning that Cdk5-deficiency could induce potent antitumor immune memory generation.
The PD-L1 is often overexpressed on tumor cells and allows cancer cells to evade immune elimination. PD-L1 binding to the PD-1 receptor at T cells halts T cell response which serves as a key immune checkpoint and ensures appropriate immune response. Tumor cells use the PD-1/PD-L1 pathway to escape the detection and elimination by the immune system. Therefore, targeting immune checkpoints such as the PD-1/PD-L1 pathway is used to overcome anti-cancer immunity resistance and has shown promising results in anti-cancer treatment (8). Of note, Dorand et al. elucidate a link between PD-L1 and Cdk5 by demonstrating that Cdk5 and PD-L1 expression co-occur in human tumors. Moreover, they observed that the basal expression as well as the interferon gamma (IFNγ)-driven upregulation of PD-L1 was reduced in tumor cells that were deficient for Cdk5 or treated with the small molecule Cdk5 inhibitor roscovitine. IFNγ is the most potent inducer of PD-L1 (9). IFNγ can also induce p35 and thereby activate Cdk5 (10,11) which was in line shown by Dorand et al. As underlying mechanism responsible for the reduced PD-L1 expression in response to IFNγ stimulation in Cdk5-deficient cells Dorand et al. observed increased levels and prolonged half-life of interferon regulatory factor-2 (IRF2) and IRF2BP2, which are negative regulators of PD-L1. IFNγ induces IRF1-driven transcription of PD-L1 (12) which is inhibited by IRF2 that suppresses the function of IRF1 by binding to the same DNA sequence and therefore inhibits the transcription of IFNγ induced genes (13,14). IRF2BP2 acts as a co-repressor with IRF2 (15). By applying a phosphoproteomic screen, Dorand et al. found that the phosphorylation of IFN2BP2 was increased in Cdk5-deficient cells and suggest that (a) kinase(s) which phosphorylates IFN2BP2 might be inhibited by Cdk5. An overview about the findings of Dorand et al. is provided by Figure 1.
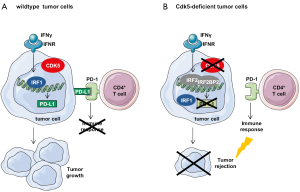
In summary, the study by Dorand et al. elucidates a new function of Cdk5 in the regulation of the PD-1/PD-L1 immune checkpoint in cancer cells and suggests the inhibition of Cdk5 as a potential target to improve the antitumor immune response.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hao Feng, MD (Experimental Surgical Research, Department of General, Visceral, Transplant, Vascular and Thoracic Surgery, Hospital of the LMU Munich, Munich, Germany).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.25). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol 2001;2:749-59. [Crossref] [PubMed]
- Feldmann G, Mishra A, Hong SM, et al. Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic cancer formation and progression through the suppression of Ras-Ral signaling. Cancer Res 2010;70:4460-9. [Crossref] [PubMed]
- Pozo K, Castro-Rivera E, Tan C, et al. The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell 2013;24:499-511. [Crossref] [PubMed]
- Liu R, Tian B, Gearing M, et al. Cdk5-mediated regulation of the PIKE-A-Akt pathway and glioblastoma cell invasion. Proc Natl Acad Sci U S A 2008;105:7570-5. [Crossref] [PubMed]
- Ehrlich SM, Liebl J, Ardelt MA, et al. Targeting cyclin dependent kinase 5 in hepatocellular carcinoma--A novel therapeutic approach. J Hepatol 2015;63:102-13. [Crossref] [PubMed]
- Dorand RD, Nthale J, Myers JT, et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 2016;353:399-403. [Crossref] [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561-84. [Crossref] [PubMed]
- Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol 2014;193:3835-41. [Crossref] [PubMed]
- Song JH, Wang CX, Song DK, et al. Interferon gamma induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of ERK1/2 pathway. J Biol Chem 2005;280:12896-901. [Crossref] [PubMed]
- Arif A, Jia J, Moodt RA, et al. Phosphorylation of glutamyl-prolyl tRNA synthetase by cyclin-dependent kinase 5 dictates transcript-selective translational control. Proc Natl Acad Sci U S A 2011;108:1415-20. [Crossref] [PubMed]
- Lee SJ, Jang BC, Lee SW, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 2006;580:755-62. [Crossref] [PubMed]
- Harada H, Fujita T, Miyamoto M, et al. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 1989;58:729-39. [Crossref] [PubMed]
- Tanaka N, Kawakami T, Taniguchi T. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol Cell Biol 1993;13:4531-8. [Crossref] [PubMed]
- Childs KS, Goodbourn S. Identification of novel co-repressor molecules for Interferon Regulatory Factor-2. Nucleic Acids Res 2003;31:3016-26. [Crossref] [PubMed]


