
Meningeal angiosarcoma: a case report and review of the literature
Introduction
Angiosarcoma is a malignant tumor that originates from vascular endothelial cells and mainly occurs in the head, face, liver, skin, and other soft tissues (1). However, primary angiosarcoma is extremely rare in the central nervous system (CNS). The epithelioid or spindle shaped tumor cells with pleomorphism show various “vasoformative” features (in cords or rudimentary, complex, anastomosing vascular channels). A primary angiosarcoma of CNS usually arises from the brain parenchyma, predominantly in the parietal lobe (2), and only a few arise from the meninges, as reported in the literature. This study reported a primary angiosarcoma arising from the meninges in an adult and reviewed relevant literature.
Case presentation
Clinical history and treatment
A 30-year-old woman was admitted to the neurosurgery department in October 2013. Without a history of head trauma or any other obvious causes, she complained of a week-long intermittent headache that worsened with the numbness of the left limb for 1 day. The neurological examination showed neck stiffness and positive Kernig’s sign. The general physical examination result was normal. No history of hypertension, diabetes, and familial inherited disease was reported. She underwent a cesarean section 1 year ago with a good recovery after that.
A magnetic resonance imaging (MRI) scan of the brain revealed a well-demarcated mass that measured 3.5 cm × 3.0 cm × 2.0 cm in the right fronto-parietal area, next to the cerebral falx. A broad based extra-axial tumor with a positive dural tail sign showed a hypointense with dotted hyperintense signal on T1 weighted image (WI), which also revealed some irregular necrotic areas and flow voids with heterogeneous enhancement on contrast.
The T2WI revealed a hyperintense with dotted hypointense signal inside. A mixed intensity with multiple tortuous flow voids and an extensive edema belt of the peripheral brain parenchyma, which was pressed by the tumor, appeared on T2 fluid attenuation inversion recovery (Figure 1A,B,C).
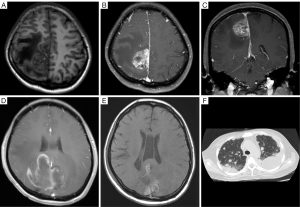
During operation, a 3.5 cm × 3.0 cm × 2.0 cm lesion was found beside the longitudinal crack next to the cerebral falx. The tumor was highly vascular, fed by many arteries from the longitudinal crack and brain. The tumor was completely removed and subjected to a pathological examination. No obvious abnormal neurologic sign was found after surgery in this patient. After surgery, the patient was started on a 5-day inductive chemotherapy (temozolomide, TMZ, 240 mg per day) and then a cycle of radiotherapy (GTV-P65.8GY/28F). She underwent a consecutive 6-week concurrent radio-chemotherapy (TMZ 240 mg; GTV2-P58.8GY/28F) accompanied by a targeted drug bevacizumab (10 mg/kg), biweekly. Thereafter, the patient received TMZ (120 mg) and bevacizumab (10 mg/kg) biweekly, 4 times in total. Three months after surgery, an MRI scan of the brain of the patient, who complained of recurrent headache and dizziness for a period of 2 months, revealed a bilateral intracranial space-occupying lesion located at the top of the fronto-parietal section adjacent to the cerebral falx (Figure 1D). It was a recurrence of angiosarcoma confirmed by a second operation and the following pathological evaluation. The patient refused further radiotherapy and chemotherapy. Two months later, the patient returned with the chief complaint of headache for 3 days. Another MRI scan of the brain showed lesions similar to the previous ones (Figure 1E). The chest and back pain appeared 2 months later, and then a chest computed tomography (CT) scan revealed multiple pulmonary metastases with bilateral pleural effusion (Figure 1F). Palliative medications were given to the patient, but she finally was in a coma and passed away.
Pathological features
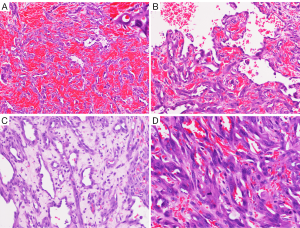
Grossly, the tumor nodule that measured 3.5 cm × 3.0 cm × 2.0 cm had soft texture with a crimson surface and cross-section. Microscopically, the tumor, covering the surface of the brain parenchyma, merged with the leptomeninges. Tumor cells showed various histological patterns such as sheets, nests, strips, blood sinus, and primitive vascular structures that were full of red blood cells and contained papillary structures inside the lumen that was lined by either spindle-shaped or epithelioid cells with marked atypia and unclear boundaries. The spindle-shaped tumor cells had fusiform and dark nuclei, whereas the epithelioid tumor cells had round or oval vesicular nuclei with basophilia, one to two small nucleoli, and shows 20 mitotic figures/10HPF. The tumor infiltrated the brain parenchyma with unclear boundaries, diffuse hemorrhage, and a large area of necrosis (Figure 2).
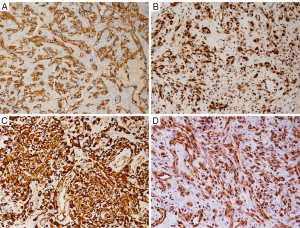
Immunohistochemical staining showed that the cells were positive for CD31 (Figure 3A), CD34, factor VIII (FVIII), vimentin, FLI-1 (Figure 3B), CD117, Olig2, S-100, nestin (Figure 3C), and ERG (Figure 3D), but negative for synaptophysin (SYN), glial fibrillary acidic protein (GFAP), D2-40, cytokeratin (CK), epithelial membrane antigen (EMA), progesterone receptor (PR), anaplastic lymphoma kinase (ALK), and CD30, desmin, and E-cadherin. The MIB-1 labeling index was 10%.
The pathological diagnosis was angiosarcoma.
Light gray tumor fragments (re-operation), which measured 6.0 cm × 6.0 cm × 2.0 cm, represented dark red soft tissue with hemorrhage and partly necrosis. the tumor had almost the same histopathology and immunophenotypes as the previous one, besides the extreme rise in the MIB-1 labeling index up to 90%.
The pathological diagnosis was angiosarcoma with massive necrosis.
Discussion
Primary intracranial angiosarcoma is a rare malignant tumor that occurs in each lobe of the brain, predominantly the parietal lobe, and is extremely rare in meninges. Approximately eight cases have been reported till date, including the one reported in this study. Of these cases, four cases were found in the brain, two in the spinal dura, another in the junction of pons and cerebellum, and the last one without clear position of the tumor (Table 1). These patients aged from 18 months to 60 years at diagnosis with a median age of 30 years, and included four males and two females with no gender information about the remaining two cases.

Full table
Clinical features
Primary angiosarcoma of CNS results in various neurological symptoms that depend on where the tumor appears and how fast it grows (2). Headache and vomiting, due to increasing intracranial pressure caused by the tumor, are the most common symptoms before a surgical resection of the tumor. If the nervous system is damaged by the tumor, corresponding neurological symptoms appear, such as seizures, numbness, and so forth.
Imaging studies can provide clues about meningeal angiosarcoma. MRI scans are characterized by irregular mixed intensity. T1WI reveals a heterogeneous hypointense signal with obvious hemorrhage, flow voids, and heterogeneous enhancement on contrast. Otherwise, a heterogeneous hyperintense signal appears on T2WI. Although a dural tail sign can be seen in common meningeal tumors, including the meningeal angiosarcoma (3,4), all the aforementioned imaging characters can help distinguish it from other meningeal tumors.
Origin and genetics
The pathogenetic mechanism of angiosarcoma may be related to many vascular growth factors. Lots of studies suggested that vascular endothelial growth factor (VEGF) and its receptors can be overexpressed in angiosarcomas, especially highly concentrated VEGF-A with its receptors found in tissues of angiosarcoma (7). Lack of VEGFR-2 expression in tumor tissues could herald a worse prognosis (8). Angiosarcoma may also be associated with the abnormal expression of TP53 (7,9), Wilms’ tumor-1, and galectin-3 (10,11).
Studies have reported K-ras gene mutations in angiosarcoma of liver and cardiac tissue (12-14). Some researchers found that about 50% of angiosarcoma had positive C-kit gene expression, but showed no activating mutations on exon 11 (juxtamembrane domain) or 17 (tyrosine kinase domain) (15-17). Immunohistochemical analysis for CD117 was positive in this case. Common chromosomal abnormalities of angiosarcoma included trisomy 5; deletions on the short arm of chromosome 7; varied abnormalities on chromosomes 8, 20, and 22; and loss of chromosome Y (18).
Pathological diagnosis
Histopathologically, the primary diagnostic component is the presence of vasoformative structures in cords or rudimentary, complex, anastomosing vascular channels cords lined with epithelioid or fusiform tumor cells with marked atypia and hyperchromatic nuclei, in different sizes and shapes. The tumor cells display papillary patterns (4), blood sinus, or original blood vessels, which indicate well differentiation, whereas solid arrangement patterns suggest poor differentiation. In some cases, large areas of a tumor can be observed that are necrotic with neoplastic infiltration into the adjacent brain tissue. Immunohistochemistry tests reveal tumor cells strongly positive for some endothelial cell markers, such as CD31, CD34, and FVIII factor, but negative for GFAP and NeuN markers. In the last few years, FLI-1, with a high specificity, has had an important role in the diagnosis of angiosarcoma. Some other researchers found that angiosarcoma showed the positive expression of nestin, which increased with the grade of a tumor (19,20).
The diagnosis of angiosarcoma was mainly based on histologic features of vascular differentiation with definite cytologic pleomorphism and immunohistochemical characteristics. It needs to be differentiated from some other meningeal tumors such as malignant meningioma and solitary fibrous tumor/hemangiopericytoma, or intracranial tumors such as gliosarcoma, using histologic primary vascular structures and immunohistochemical characteristics. Malignant meningiomas could occur in cancerous, melanomatous, or high-grade sarcomatoid patterns but without expressing CD31 and FVIII factor. The solitary fibrous tumor/hemangiopericytoma also featured the typical well-differentiated antler-shaped branching vessels with the uneven thickness of walls without expressing CD31 and FVIII factor. In gliosarcomas, mesenchymal and glial differenciation can be found, while it is absent in the case of angiosarcoma. Another important differential diagnosis includes metastatic angiosarcoma to the CNS that often has a history of primary tumors.
Treatment and prognosis
Primary treatment for angiosarcoma is surgical resection. An adjuvant radiotherapy or chemotherapy may yield some effects in a subset of patients when total resection of a tumor is hardly achieved. Temozolomide is effective for sarcoma (21), particularly as an option for CNS sarcomas because it can pass through the blood-brain barrier. Bevacizumab, as a VEGF inhibitor, has been used successfully in some recurrent glioblastomas (22). However, its effect on primary CNS angiosarcoma has not been reported. In most instances, combination chemotherapy has not been confirmed any more effective than single-agent therapy (21). Although radiation therapy is reported to be effective against angiosarcoma of the bone or metastatic cerebral angiosarcoma, findings on the effectiveness of radiotherapy against primary cerebral angiosarcomas are still controversial (5). Prognosis is poor considering the malignant nature of the tumor. In the series reported by Mena et al. (2), the median survival time in the five patients was 8 months, and the tumors were located in the cerebral hemispheres. Among the primary CNS angiosarcomas, primary meningeal angiosarcomas are even rarer. Only seven cases have been reported in the literature. The prognosis of the meningeal angiosarcoma is very poor.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Fujian Province, P.R.C. (Grant No. 2014J01413).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer 1995;75:989-96. [Crossref] [PubMed]
- Mena H, Ribas JL, Enzinger FM, et al. Primary angiosarcoma of the central nervous system. Study of eight cases and review of the literature. J Neurosurg 1991;75:73-6. [Crossref] [PubMed]
- Hackney JR, Palmer CA, Riley KO, et al. Primary central nervous system angiosarcoma: two case reports. J Med Case Rep 2012;6:251. [Crossref] [PubMed]
- Guode Z, Qi P, Hua G, et al. Primary cerebellopontine angle angiosarcoma. J Clin Neurosci 2008;15:942-6. [Crossref] [PubMed]
- Russell DS, Rubinstein LJ. Pathology of Tumors of the Nervous System. 5th ed. Baltimore: Lippincott Williams & Wilkins, 1989:657-8.
- Kristoferitsch W, Jellinger K. Multifocal spinal angiosarcoma after chordotomy. Acta Neurochir (Wien) 1986;79:145-53. [Crossref] [PubMed]
- Zietz C, Rössle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol 1998;153:1425-33. [Crossref] [PubMed]
- Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol 2008;97:74-81. [Crossref] [PubMed]
- Naka N, Tomita Y, Nakanishi H, et al. Mutations of p53 tumor-suppressor gene in angiosarcoma. Int J Cancer 1997;71:952-5. [Crossref] [PubMed]
- Ueda T, Oji Y, Naka N, et al. Overexpression of the Wilms' tumor gene WT1 in human bone and soft-tissue sarcomas. Cancer Sci 2003;94:271-6. [Crossref] [PubMed]
- Johnson KD, Glinskii OV, Mossine VV, et al. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia 2007;9:662-70. [Crossref] [PubMed]
- Przygodzki RM, Finkelstein SD, Keohavong P, et al. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest 1997;76:153-9. [PubMed]
- Weihrauch M, Bader M, Lehnert G, et al. Mutation analysis of K-ras-2 in liver angiosarcoma and adjacent nonneoplastic liver tissue from patients occupationally exposed to vinyl chloride. Environ Mol Mutagen 2002;40:36-40. [Crossref] [PubMed]
- Garcia JM, Gonzalez R, Silva JM, et al. Mutational status of K-ras and TP53 genes in primary sarcomas of the heart. Br J Cancer 2000;82:1183-5. [PubMed]
- Miettinen M, Sarlomo-Rikala M, Lasota J. KIT expression in angiosarcomas and fetal endothelial cells: lack of mutations of exon 11 and exon 17 of C-kit. Mod Pathol 2000;13:536-41. [Crossref] [PubMed]
- Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol 2002;117:188-93. [Crossref] [PubMed]
- Komdeur R, Hoekstra HJ, Molenaar WM, et al. Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin Cancer Res 2003;9:2926-32. [PubMed]
- Wong KF, So CC, Wong N, et al. Sinonasal angiosarcoma with marrow involvement at presentation mimicking malignant lymphoma: cytogenetic analysis using multiple techniques. Cancer Genet Cytogenet 2001;129:64-8. [Crossref] [PubMed]
- Mokrý J, Nemecek S. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biol (Praha) 1998;44:155-61. [PubMed]
- Mokrý J, Nemecek S. Cerebral angiogenesis shows nestin expression in endothelial cells. Gen Physiol Biophys 1999;18:25-9. [PubMed]
- Penel N, Van Glabbeke M, Marreaud S, et al. Testing new regimens in patients with advanced soft tissue sarcoma: analysis of publications from the last 10 years. Ann Oncol 2011;22:1266-72. [Crossref] [PubMed]
- Seystahl K, Wiestler B, Hundsberger T, et al. Bevacizumab alone or in combination with irinotecan in recurrent WHO grade II and grade III gliomas. Eur Neurol 2013;69:95-101. [Crossref] [PubMed]


