
Sugar intake from sugar-sweetened beverage among cancer and non-cancer individuals: the NHANES study
Introduction
It has been estimated that there are approximately 14.5 million cancer survivors in the United States (1). Although 5-year survival rates for early stage colorectal, breast, and prostate cancer currently exceed 90% and are increasing, cancer survivors are still at greater risk for second malignancies and co-morbid conditions such as other chronic diseases (2,3). Growing scientific evidence shows that health-related risk behaviors including tobacco use, alcohol use, physical activity, healthy dietary patterns, and weight control may impact health among cancer survivors (2-7). Sugar intake or sugar-sweetened beverage (SSB) consumption has been demonstrated to have a positive association with obesity, diabetes, and cardio-metabolic diseases, as well as some cancers (6,7). Therefore, SSB consumption among cancer survivors will be an important issue as survival rates increase due to improvements in treatment and health care. Thus, it is of particular interest to examine SSB consumption behaviors among cancer survivors.
SSBs include soda, fruit drinks, punches, sports drinks, tea and other beverages that contain added caloric sweeteners. The sugar content of SSBs is typically 10–15 g per 100 mL (8). Over the last three decades in the United States, high fructose corn syrup (HFCS) has largely replaced sucrose as a major sweetener, and HFCS is largely consumed through soda (9). The American Heart Association (AHA) recommends a consumption goal of no more than 450 kilocalories (kcal) of SSB or fewer than three 12 ounce cans of soda per week (8). SSBs are the major source of added sugar in the western diet and greatly contribute to daily total energy in the diets of children and adolescents in the United States (10,11). Additionally, data from the 2013 Behavioral Risk Factor Surveillance System (BRFSS) indicates that socioeconomic status disparities might contribute to differences in SSB consumption patterns. A study by Park et al. found that SSB consumption prevalence was highest among non-Hispanic black males (39.9%), unemployed adults (34.4%), and persons with less than a high school education (42.4%) (12).
Research also indicates that SSBs can have deleterious effects on weight status, and SSB intake may be associated with poor diet quality. Added sugars from SSBs contribute zero nutrients but add calories that can lead to extra pounds, and consumption of SSBs has been shown to significantly increase the rate of overweight and obesity (13,14). Furthermore, obesity independently has been associated with a range of cancer types, such as liver, prostate, colon, and endometrial (15). Additionally, the consumption of SSBs is associated with overall poorer diet quality and increased fast food consumption, as well as inadequacy of multiple nutrient intakes (16-18). A cross-sectional laboratory study found that SSB intake was inversely associated with calcium and vitamin D intake, which may be detrimental as previous research has shown that calcium may be protective against obesity (17). Research also indicates that the sweeteners found in SSBs may negatively impact cardio-metabolic health. In one randomized controlled crossover trial in healthy young men, participants were assigned to six different intervention groups for which they consumed 600 mL of SSBs with varied amounts of fructose, sucrose, and/or glucose daily. Results showed that after only 3 weeks of intervention, fasting glucose and C-reactive protein were significantly elevated among all of the trial arms; and participants who drank fructose-containing SSBs experienced a change towards a more atherogenic subclass distribution of low-density lipoprotein (LDL) (9).
Evidence for how sugar intake or SSB consumption affects cancer risk, however, is limited and unclear (2,3,5). Slattery et al. analyzed data from a population-based case-control study (n=1,993 cases and 2,410 controls) to examine the associations between dietary sugars, foods containing high level of sugars, and dietary glycemic index and colon cancer. Results showed that dietary sugars, especially diets high in simple carbohydrates relative to complex carbohydrates, increased risk of colon cancer, possibly through their impact on plasma glucose levels (6). Similarly, a study of endometrial cancer risk and sugar intake found that higher intake of SSBs and sugars was associated with an increased risk of type I, though not type II, endometrial cancer (19). On the contrary, Bao et al. examined 487,922 men and women aged 50–71 years and found that consumption of added sugar or of sugar-sweetened foods and beverages was not associated with overall risk of pancreatic cancer (5). A separate study by King et al. evaluated ovarian cancer risk in relation to sugary foods and beverages, and total and added sugar intakes in a population-based case-control study and found no evidence of an association between consumption of sugary foods and beverages and cancer risk. However, there was a suggestion of increased risk associated with SSB intake (servings per 1,000 kcal; OR =1.63, 95% CI: 0.94–2.83) (7). Given current evidence, the importance of SSB intake on cancer risk may vary by type of cancer, and more research is required on this topic to better understand these relationships.
Currently, little research exists examining SSB intake in cancer patients or cancer survivors. One recent longitudinal study assessed the association between SSB consumption on cancer recurrence and mortality in 1,011 stage III colon cancer patients. Researchers measured SSB intake 4 months after surgery and again 6 months after completion of chemotherapy treatment. Results showed that higher SSB intake was associated with a significantly increased risk of cancer recurrence and mortality among patients (20). To our knowledge, no other studies have examined SSB intake in cancer survivors. Thus, the objective of this study is to closely evaluate the risk factors of sugar consumption (total sugar intake and sugar binge behavior) from SSBs among non-cancer individuals and cancer survivors.
Methods
Study population
A total of 22,182 adults aged ≥20 years old and with valid responses in the cancer and SSB related questions from the National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics of Centers for Disease Control and Prevention (CDC), were included in this study. NHANES is a nationally representative survey designed for assessing health and nutritional status of the resident civilian non-institutionalized United States population (21). Five consecutive 2-year survey cycle data sets were combined (2003–2004, 2005–2006, 2007–2008, 2009–2010 and 2011–2012) to evaluate the impact of cancer status’ and other risk factors on sugar intake from SSBs.
Measurements
SSBs were defined as any of the following drinks that are sweetened before purchase: sodas, fruit-flavored drinks, sweetened fruit juices, sport drinks, energy drinks, sweetened teas and coffees and other sugar-sweetened drinks. In addition, any above drinks which were unsweetened before purchase and added sugar before consumption were also considered as SSBs in this study. The food codes from the United States Department of Agriculture (USDA) for the beverage groups were used to identify the SSBs (22). The SSB related measurements were calculated based on the average of two 24-hour dietary recall interviews in NHANES. The first interview was collected in-person in the mobile examination center (MEC) and the second interview was collected by telephone 3 to 10 days after the first interview.
SSB sugar binge was defined as average grams of sugar from SSB consumption per occasion per day. This measure does not attempt to classify behavior as regular versus irregular (e.g., binge versus non-binge). Rather, the advantage of SSB sugar binge is that it takes SSB frequency into consideration and measures consumption of sugar from SSB in a short period of time. Thus, it offers insight into patterns of sugar intake from SSB rather than total intake alone. Our primary outcome was sugar intake from SSB consumption: low (<80 g per day) and high (≥80 g per day). The AHA’s recommendation of added sugars allowance is no more than 24 g of sugar for women and 36 g of sugar for men per day (8). Our 80 g cutoff for high sugar intake was based on approximately twice the AHA’s recommendation of added sugars allowance for men. The 80 g cutoff is also approximately equal to the amount of sugar found in two cans of soda (~40 g sugar per can). For non-cancer population, the amount of sugar from SSB per day for the top 25% subjects is 75.2 g (25%: 19.0 g and median: 39.6 g). In addition to sugar intake from SSB consumption, other SSB indicators included SSB intake (yes/no), SSB consumption count per day, and SSB sugar binge per day. SSB sugar binge was defined as average grams of sugar from SSB consumption per occasion per day.
Cancer status was defined based on the question: “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” Among cancer patients, seven common cancer types were also evaluated: prostate, breast, cervix, colon, melanoma, uterus, and other skin cancer. This disease classification is based on the primary cancer site.
The participants’ demographic characteristics included age, gender, race (White, Black, and other), education (< high school, high school, > high school), and poverty income ratio. Poverty income ratio, with a range of 0–5, is the ratio of family income to their appropriate poverty threshold. Ratios below one indicate that the family income is below the official definition of poverty. Obesity and smoking status were also considered. The sub-categories of these factors are listed in Table 1.
Full table
Statistical analysis
The participants’ demographic, smoking, obesity and cancer characteristics by sugar intake from SSBs status were summarized using descriptive statistics. The differences of categorical variables in the two sugar intake from SSBs groups (low vs. high) were assessed using the Rao-Scott chi-square with an adjusted F statistic; and the differences of continuous variables were tested using t-tests. In order to evaluate factors associated with high sugar intake from SSB (≥80 g) adjusting for other factors, a logistic regression model with the appropriate sampling weights was applied (using PROC SURVEYLOGISTIC in SAS) (SAS Institute Inc., Cary, North Carolina, USA). The selection of variables included in the final multivariable model was based on whether the variable showed a statistically significant result (P value <0.05) in the univariate models. We further compared SSB intake (yes/no), SSB consumption count per day, sugar intake from SSB (numeric scale) and SSB sugar binge per day between non-cancer and cancer individuals. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc.). The weighting analytical methods followed the instructions provided on the CDC website (23). A P value of less than 0.05 was considered statistically significant.
Results
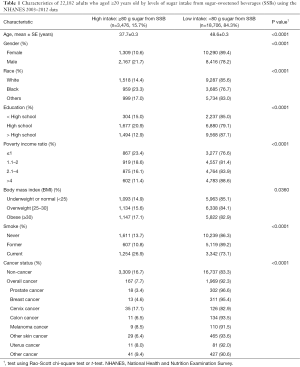
There were a total of 22,182 adults aged ≥20 years old in the NHANES 2003–2012. Table 1 shows the demographic and behavioral characteristics and cancer status by the two sugar intake from SSB groups [high (≥80 g) vs. low (<80 g) sugar per day]. For the overall study population, 15.7% had high sugar intake from SSBs.
Non-cancer individuals had a higher sugar intake from SSBs than cancer survivors (16.7% vs. 7.7%, respectively). As shown in Table 1, individuals with high sugar intake from SSBs were younger than those with low sugar intake from SSBs (mean, 37.7 vs. 48.6 years old). In addition, rates of high sugar intake from SSBs were greatest in males (21.7%), Blacks (23.3%), participants with high-school education level (20.9%), low income (poverty income ratio ≤1) (23.4%), obese (17.1%), current smokers (26.9%), and without cancer history (16.7%) or with cervical cancer (17.1%). Cervical cancer survivors had the highest rate of high sugar intake from SSBs (17.1%) and prostate cancer survivors had the lowest rate of high sugar intake from SSBs (3.4%). The rates of high sugar intake from SSBs for colon and breast cancer were 6.5% and 4.6%, respectively. The rates of other cancer types are listed in Table 1.
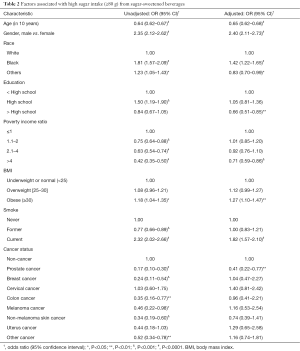
Factors associated high sugar intake from SSBs were assessed using univariate and multivariable logistic regression models (Table 2). The unadjusted model results shown in Table 2 are similar to the results in Table 1. In the unadjusted univariate models, prostate (OR =0.17, P<0.0001), breast (OR =0.24, P<0.001), colon (OR =0.35, P<0.01), melanoma (OR =0.46, P<0.05), non-melanoma (OR =0.34, P<0.001), and other cancer types (OR =0.52, P<0.01) were less likely to have high sugar intake from SSBs compared with the non-cancer individuals.
Full table
For adjusted comparisons of sugar intake from SSBs between cancer and non-cancer individuals, the multivariable weighted logistic regression models was conducted. After controlling for the factors listed in Table 2, the association between high sugar intake from SSBs and cancer status became insignificant except for prostate cancer (vs. non-cancer, OR =0.41, P<0.01). In addition, the older participants were less likely to have high sugar intake from SSBs (OR in a 10-year increment =0.65, P<0.0001). Smoking status and obesity were significantly associated with high sugar intake from SSBs after adjusting for demographic factors. Current smokers (vs. never smokers, OR =1.82, P<0.001) and obese individuals (vs. normal weight, OR =1.27, P<0.01) were more likely to have high sugar intake from SSBs.
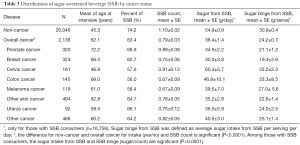
The distributions of SSB related factors among non-cancer individuals and cancer survivors were also explored. Several SSB related measurements [SSB consumption (%), SSB count, sugar intake from SSBs and sugar binge per occasion] were evaluated. Results in Table 3 show more non-cancer individuals responded “yes” to having consumed SSBs than cancer survivors (74.2% vs. 63.4%). Non-cancer individuals consumed more SSBs than cancer survivors (mean count per day 1.10 vs. 0.79). Among those who consumed SSBs, non-cancer individuals had more sugar from SSBs per day than cancer survivors (mean, 54.9 vs. 38.4 g/day). In addition, non-cancer individuals consumed more sugar per SSBs than cancer survivors (mean, 30.9 vs. 24.2 g/serving). We did further analyses to evaluate SSBs related factors among survivors with different cancer types. Among cancer survivors, prostate cancer survivors had the highest proportion with SSB consumption (68.9%) and had relatively high SSB count per day (mean, 0.89); however, prostate cancer survivors had lower sugar intake from SSBs (mean, 34.5 g/day) and lower sugar binge from SSBs (mean, 21.1 g/occasion) than other cancer survivors. Cervical cancer survivors consumed more SSBs (mean count, 0.91), more sugar from SSBs per day (mean, 60.4 g/day), and had higher SSB sugar binge (mean, 33.2 g/occasion) than other cancer survivors, although relatively few cervical cancer survivors consumed SSB (57.6%).
Full table
Discussion
This study showed that the prevalence of SSB consumption and sugar intake from SSBs among cancer survivors are lower than among non-cancer individuals. After adjusting for age, there was no significant difference of sugar intake from SSBs between cancer and non-cancer individuals. For the total sample, individuals who were younger, male, Black, poorer, had a high school education level, were a current smoker, or were obese were more likely to have high sugar intake (≥80 g sugar) from SSBs.
Added sugar is not recommended for consumption by the Food and Nutrition Board and the Institute of Medicine (24). SSBs are the major source of added sugar and greatly contribute to daily total energy in the diets of children and adolescents in the United States (10,11). However, people are not usually aware of how much sugar they consume from SSBs. The AHA recommends that no more than half of daily discretionary calories come from added sugars (8). For example, AHA’s recommended added sugars allowance for most American women is no more than 100 kcal per day, or about 6 teaspoons (24 g) of sugar. For most American males, AHA suggests no more than 150 kcal per day, or about 9 teaspoons (36 g). As a reference, one 12-ounce can of regular soda contains approximately 8 teaspoons of sugar, or 130 kcal and zero nutrition. In other words, 1 can of soda would account for more than 88% of an adult male’s and 133% of an adult female’s whole day recommendations for added sugar consumption.
Our study found age was the leading factor associated with high sugar intake from SSBs. The odds of having high sugar intake from SSBs decreased as age increased. In unadjusted models, several cancer types were statistically significantly associated with high sugar intake from SSBs. However, when adjusting for age, these associations became insignificant for all cancers except prostate cancer. This result may explain why some studies (7,25) did not find a significant relationship between cancer status and SSB consumption; when age or age-cancer interaction was not considered, the relationship appeared insignificant. In the present study, we observed that there is a significant interaction of age and cancer (P=0.012) associated with high sugar intake from SSB. This indicates the age impact on sugar intake from SSBs is different for cancer (OR =0.53, 95% CI: 0.45–0.62, P<0.0001) versus non-cancer individuals (OR in a 10-year increment =0.65, 95% CI: 0.63–0.68, P<0.0001). The sugar intake from SSBs decreased more as age increased for cancer survivors compared with non-cancer individuals. Overall, the rate of high sugar intake from SSBs among cancer survivors is lower than the non-cancer population. However, this may be explained by the finding that, individuals with high sugar intake from SSBs were younger than those with low sugar intake from SSBs, while the majority of cancer survivors were older. In addition, the different age distributions in the non-cancer individuals and cancer survivors (mean age =45.3 and 62.1 years old, respectively) supports this hypothesis.
Similarly, the mean ages of prostate, breast, colon, melanoma, other skin cancer, uterus and other cancer survivors were higher than non-cancer individuals. The associations between cancer types and SSB behavior observed in our study were consistent with the findings from the previous studies. Recent studies found that higher intake of SSB and sugars was associated with an increased risk of cancer or recurrence. For example, one study found that higher intake of SSB and sugars was associated with an increased risk of type I, but not type II, endometrial cancer (19). Another study assessed the association between SSB consumption on cancer recurrence and mortality in 1,011 stage III colon cancer patients and found that higher SSB intake was associated with a significantly increased risk of cancer recurrence and mortality (20). The sugar intake from SSBs among individuals with cervical cancer history was much higher in our study (60 g/day) compared to other cancer survivors who consumed only around 30–40 g/day. However, the mean age of cervical cancer survivors was younger (mean, 46 years old) than other cancer survivors. By evaluating the differences in age-adjusted sugar intake for specific cancer sub-groups, we can conclude that while age was an important factor, the relationship appears insignificant. This age difference between the two groups may explain the association of sugar intake from SSBs and cancer.
In addition to these findings of the effect of age on the relationship between SSBs and other factors, we observed that the impacts of obesity and smoking status were significantly associated with high sugar intake from SSBs. These findings are consistent with the previous research (26-29). Compared to underweight or normal weight individuals, individuals who were obese were more likely to have high sugar intake from SSBs. It is well documented that obesity is related to excess energy intake. SSBs are the major source of added sugar and greatly increase the total energy in diets (10,11). Smoking and obesity are two major leading causes of morbidity and mortality for most cancers and chronic diseases (29-32). Previous studies indicate that nicotine increases energy expenditure and could reduce appetite, which may explain why smokers tend to have lower body weight than nonsmokers. It may also explain why smoking cessation is frequently followed by weight gain (33). However, studies also show that more smokers tend to have higher rates overweight or obese than nonsmokers (34). One potential reason is risk behavior pattern, which likely reflects a clustering of risky behaviors together. For example, studies showed that smoking, alcohol drinking, physical inactivity, and poor dietary habits are usually clustered together (35-37). In this study, results showed that smoking is associated with high sugar intake from SSBs. Compared to never smokers, current smokers were more likely to have high sugar intake from SSBs.
There are several limitations to this study. First, cancer status in this study may have been biased because it was self-reported without validation using medical records. Second, sugar intake from SSBs among cancer survivors might not be generalized to the whole cancer population because hospitalized cancer patients were not considered. Third, this study focuses on evaluating the impact of cancer status and other risk factors on sugar intake from SSB and not sugar from food intake. Previous studies show that SSB intake is positively associated with obesity after controlling for total energy intake (including sugar intake from food); therefore, in the current investigation we focused on sugar intake from SSB specifically. Fourth, although all analyses were weighted to account for the complex sampling design applied in NHANES, some subgroups (such as specific cancer patients) may not represent the whole United States population due to survey limitations.
Conclusions
This study investigated the distribution of sugar intake from SSBs and examined the association of SSB related factors among non-cancer individuals and cancer survivors. In general, cancer survivors had lower prevalence of sugar intake from SSBs than non-cancer individuals. However, this cancer group difference was not significant after adjusting for other factors. In addition, individuals who had high sugar intake (≥80 g sugar) from SSBs were younger, male, Black, had education levels at/below high school, low income, obese, current smokers and non-cancer individuals. This study provides the valuable information that age is an important factor in discussing the association between cancer and SSBs. The lower overall rate of sugar intake from SSBs in cancer survivors compared to non-cancer individuals was primarily due to the majority of cancer survivors being elderly. SSB consumption behavior varies across cancers and may be related to age. Intervention programs that aim to reduce SSB or added sugar consumption among cancer survivors should primarily focus on lower socio-economic status young males, both non-cancer and cancer survivors. As suggested by “The CDC Guide to Strategies for Reducing the Consumption of Sugar-Sweetened Beverages” (38), “Include screening and counseling about SSB consumption as part of routine medical care.” Custom intervention of decreasing sugar consumption from SSBs should be conducted for both non-cancer individuals and cancer survivors in communities and the medical care system. Moreover, developing programs to reduce SSB consumption for cervical cancer survivors, especially those in younger age groups, is of high priority.
Acknowledgments
We thank anonymous reviewers for their valuable comments on this manuscript.
Funding: This research was supported by the School of Public Health, LSUHSC-New Orleans, the LSUHSC SPH Jim Finks Endowed Chair in Health Promotion Research Fund, the Louisiana Cancer Research Center, the Nutrition Obesity Research Center at Pennington Biomedical Research Center, NIDDK (CNRU) 1P30 DK072476, R01 HD49046; NIMHD 5U54MDO08176-02.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Social Behavioral and Genetic Risk factors for Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.42). The series “Social Behavioral and Genetic Risk factors for Cancer” was commissioned by the editorial office without any funding or sponsorship. TST and HYL served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The NHANES data was approved by the National Center for Health Statistics Institutional Review Board/Ethics Review Board, and written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts and Figures 2016. Available online: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf
- Genkinger JM, Li R, Spiegelman D, et al. Coffee, tea, and sugar-sweetened carbonated soft drink intake and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Cancer Epidemiol Biomarkers Prev 2012;21:305-18. [Crossref] [PubMed]
- Bradshaw PT, Sagiv SK, Kabat GC, et al. Consumption of sweet foods and breast cancer risk: a case-control study of women on Long Island, New York. Cancer Causes Control 2009;20:1509-15. [Crossref] [PubMed]
- Lewis CM, Wolf WA, Xun P, et al. Racial differences in dietary changes and quality of life after a colorectal cancer diagnosis: a follow-up of the Study of Outcomes in Colorectal Cancer Survivors cohort. Am J Clin Nutr 2016;103:1523-30. [Crossref] [PubMed]
- Bao Y, Stolzenberg-Solomon R, Jiao L, et al. Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr 2008;88:431-40. [PubMed]
- Slattery ML, Benson J, Berry TD, et al. Dietary sugar and colon cancer. Cancer Epidemiol Biomarkers Prev 1997;6:677-85. [PubMed]
- King MG, Olson SH, Paddock L, et al. Sugary food and beverage consumption and epithelial ovarian cancer risk: a population-based case-control study. BMC Cancer 2013;13:94. [Crossref] [PubMed]
- American Heart Association. Added Sugars. Available online: http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Nutrition/Added-Sugars_UCM_305858_Article.jsp#.Vy4L9ORa3hW
- Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479-85. [Crossref] [PubMed]
- Park S, Blanck HM, Sherry B, et al. Factors associated with sugar-sweetened beverage intake among United States high school students. J Nutr 2012;142:306-12. [Crossref] [PubMed]
- Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc 2010;110:1477-84. [Crossref] [PubMed]
- Park S, Xu F, Town M, et al. Prevalence of Sugar-Sweetened Beverage Intake Among Adults--23 States and the District of Columbia, 2013. MMWR Morb Mortal Wkly Rep 2016;65:169-74. [Crossref] [PubMed]
- Hafekost K, Mitrou F, Lawrence D, et al. Sugar sweetened beverage consumption by Australian children: implications for public health strategy. BMC Public Health 2011;11:950. [Crossref] [PubMed]
- Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274-88. [PubMed]
- Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol 2013;2013:697521.
- Marshall TA, Eichenberger Gilmore JM, Broffitt B, et al. Diet quality in young children is influenced by beverage consumption. J Am Coll Nutr 2005;24:65-75. [Crossref] [PubMed]
- Keller KL, Kirzner J, Pietrobelli A, et al. Increased sweetened beverage intake is associated with reduced milk and calcium intake in 3- to 7-year-old children at multi-item laboratory lunches. J Am Diet Assoc 2009;109:497-501. [Crossref] [PubMed]
- Collison KS, Zaidi MZ, Subhani SN, et al. Sugar-sweetened carbonated beverage consumption correlates with BMI, waist circumference, and poor dietary choices in school children. BMC Public Health 2010;10:234. [Crossref] [PubMed]
- Inoue-Choi M, Robien K, Mariani A, et al. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev 2013;22:2384-94. [Crossref] [PubMed]
- Fuchs MA, Sato K, Niedzwiecki D, et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS One 2014;9:e99816 [Crossref] [PubMed]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes.htm
- Mesirow MS, Welsh JA. Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of US children: National Health and Nutrition Examination Survey 2001-2010. J Acad Nutr Diet 2015;115:559-66.e4. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Specifying Weighting Parameters. Available online: http://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/intro.htm
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press, 2006.
- Burley VJ. Sugar consumption and human cancer in sites other than the digestive tract. Eur J Cancer Prev 1998;7:253-77. [Crossref] [PubMed]
- Su P, Hong L, Sun H, et al. Age plays an important role in the relationship between smoking status and obesity risk: a large scale cross-sectional study of Chinese adults. Int J Clin Exp Med 2015;8:18894-906. [PubMed]
- Larson N, Dewolfe J, Story M, et al. Adolescent consumption of sports and energy drinks: linkages to higher physical activity, unhealthy beverage patterns, cigarette smoking, and screen media use. J Nutr Educ Behav 2014;46:181-7. [Crossref] [PubMed]
- Maatoug J, Harrabi I, Hmad S, et al. Clustering of risk factors with smoking habits among adults, Sousse, Tunisia. Prev Chronic Dis 2013;10:E211 [Crossref] [PubMed]
- García-Jiménez C, Gutiérrez-Salmerón M, Chocarro-Calvo A, et al. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer 2016;114:716-22. [Crossref] [PubMed]
- Tseng TS, Lin HY, Moody-Thomas S, et al. Who tended to continue smoking after cancer diagnosis: the national health and nutrition examination survey 1999-2008. BMC Public Health 2012;12:784. [Crossref] [PubMed]
- Golabek T, Bukowczan J, Szopinski T, et al. Obesity and renal cancer incidence and mortality--a systematic review of prospective cohort studies. Ann Agric Environ Med 2016;23:37-43. [Crossref] [PubMed]
- Patra J, Maher YI, Mishra S, et al. Effects of body mass index, tobacco smoking, alcohol drinking and solid fuel use on the risk of asthma: Individual Participant Data (IPD) meta-analysis of 175 000 individuals from 51 nationally representative surveys. BMJ Open Respir Res 2016;3:e000121 [Crossref] [PubMed]
- Walker JF, Kane CJ. Effects of body mass on nicotine-induced thermogenesis and catecholamine release in male smokers. Sheng Li Xue Bao 2002;54:405-10. [PubMed]
- Watanabe T, Tsujino I, Konno S, et al. Association between Smoking Status and Obesity in a Nationwide Survey of Japanese Adults. PLoS One 2016;11:e0148926 [Crossref] [PubMed]
- Lohse T, Rohrmann S, Bopp M, et al. Heavy Smoking Is More Strongly Associated with General Unhealthy Lifestyle than Obesity and Underweight. PLoS One 2016;11:e0148563 [Crossref] [PubMed]
- Chiolero A, Faeh D, Paccaud F, et al. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 2008;87:801-9. [PubMed]
- Tseng TS, Lin HY. Gender and age disparity in health-related behaviors and behavioral patterns based on a National Survey of Taiwan. Int J Behav Med 2008;15:14-20. [Crossref] [PubMed]
- Sugar-Sweetened Beverage Guide. The CDC Guide to Strategies for Reducing the Consumption of Sugar-Sweetened Beverages. Available online: http://www.cdph.ca.gov/SiteCollectionDocuments/StratstoReduce_Sugar_Sweetened_Bevs.pdf


