
Commentary on “A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors”
The field of adoptive immunotherapy has grown from the early days of a few academic groups administering cytotoxic lymphocytes (CTL) or tumor infiltrating lymphocytes (TIL), to the use of genetically engineered T cells being developed as drug products by multinational pharmaceutical companies. The first demonstration of the effective use of gene engineered T cells was reported in 2006 when T-cell receptor (TCR) engineered cells were shown to mediate cancer regression in 2 of 13 patients with malignant melanoma (1). In the subsequent years, several groups spearheaded the clinical developed an alternative antigen targeting technology based on chimeric antigen receptors (CAR), which mediate tumor antigen recognition via ligand binding domains (generally antibodies) and bypass the TCR complex by being fusion proteins with T cell signaling domains such as CD3zeta (2-4). CARs based on anti-CD19 mAbs have been shown to mediate durable cancer regression in leukemia and lymphoma in multiple clinical trials and it is these results that most stimulated the excitement of this therapy as a new treatment modality for cancer (5-7).
While there has been consistent success in the application of CAR therapy in CD19-expressing hematological malignancies, there has not been reproducible success in the application of CAR technology in solid cancers. There are most certainly multiple reasons for the lack of effective tumor treatment by CAR engineered T cells (CART) in solid malignancies, with the tumor microenvironment the most widely proposed, and the subject of the research reported in Liu et al. Just as tumors vary from type to type (e.g., breast vs. colon), there is no one universal tumor microenvironment. While tumor microenvironments vary greatly, there are some consistent themes that may permit researchers to focus efforts on the most prevalent inhibitory factors. The immune system has a series of checkpoints that dampen the immune response and restore balance post-antigen exposure. One of the most widely studied systems is the programmed death 1 (PD-1) receptor interaction with its’ two major ligands PD-L1 and PD-L2. Antibodies targeting the PD-1/PD-L1 proteins have been studied in several clinical trials and have demonstrated to mediate responses in several solid tumor histologies (e.g., melanoma, non-small cell lung cancer) (8,9). T cell checkpoint blockade is the focus of the work by Liu et al. Genetic engineering has the potential to endow cells with novel functions using synthetic biology. Liu et al. apply the concept of synthetic biology in CART cells using what they describe as a “switch receptor complex”.
The idea of switch receptors is to produce a chimeric molecule that combines a ligand binding domain with an alternative signaling domain and this approach has been reported for a number of different constructs (10,11). In the specific example investigated in Liu et al., the investigators fused the extracellular domains of PD-1 to the transmembrane and intracellular signaling domains of CD28. This approach was initially reported by others and expanded in this report using more detailed in vivo modeling to demonstrate increase efficacy when combined with CART cells (12-14), as shown in Figure 1, when PD-1 normally binds one of its ligands in signals through the Smad proteins to blunt T cell signaling. CD28, on the other hand, is one of the main TCR co-stimulator receptors and upon engagement of its ligands (e.g., CD80) boosts the T cell response through a number of positive signaling events resulting in enhanced proliferation and cytokine production. By using a PD-1/CD28 switch receptor fusion protein, it is possible to turn the negative signal normally propagated by the PD-1/PD-L1 interaction, into a positive signal that a T cell normally receives through CD28/CD80 co-stimulation.
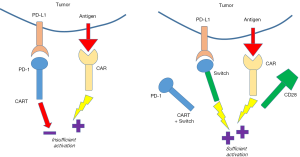
Liu et al. use three gene transfer methods to test this approach, RNA electroporation, gamma-retroviral vector, and lentiviral vector mediated gene transfer and they combined these methods in a number of antigen targeting systems with CARs recognizing CD19, PSCA, and mesothelin. RNA electroporation can quickly introduce most any protein into a cell and the co-electroporation of a CD19 CAR and the switch receptor was performed to test the impact on T cell recognition of tumor cell lines expressing both CD19 and PD-L1. In the case where the CAR alone engineered T cells encounter PD-L1 expressing targets, they produced less cytokine and showed reduced tumor cell killing than when cultured with PD-L1-negative cell lines. In the case of the CAR-switch receptor co-expressing T cells, this reduction in effector T cell responses was not observed. While RNA electroporation can be used to rapidly evaluate synthetic biology approaches, the amount of gene expression afforded by the method is generally much higher than viral-vector mediated gene transfer methods. In the second and third figures in their report, Liu et al. use gamma-retroviral and lentiviral vectors (for anti-PSCA and anti-mesothelin CARs, respectively) and reproduce the findings of the CD19 CAR switch receptor RNA electroporation experiment by demonstrating the lack of blocking activity when CART cells encounter tumor cell lines expressing PD-L1. Moreover, when comparing the activity of switch receptor-engineered T cells to CAR alone T cells, data suggest that these cells are more active when encountering PD-L1 expressing target cells.
In a last series of experiments, Liu et al. explore the biology of CAR plus switch receptor engineered T cells in tumor xenograft models. These models used well established tumors (unlike previous reports) and are a typical assay used in the CART field to validate in vitro biology. In both PD-L1 expressing mesothelin and PSCA expressing tumor models significantly better anti-tumor activity was observed when mice were administered T cells engineered with both the CARs and the switch receptor. What may be of particular interest to the application of these approaches in cancer patients were the findings on cell phenotype and persistence. PD-1 is not the only inhibitory receptor involved in control T cell activity, proteins Lag3 and Tim3 have also been associated with decreased T cell functions (15). When Liu et al. analyzed T cells from mice that received the CAR switch combination, there were fewer T cells co-expressing these additional exhaustion markers. Although the precise biology behind the co-expression of inhibitory T cell markers is still being worked out, it is clear that these markers can be co-expressed and cells expressing multiple markers are more impaired than cells expressing PD-1 alone. Finally these investigators also report that the number of TIL in mice receiving the CAR plus switch receptor engineered T cells were greater than TIL from animals that received only the CART. Whether this resulted from increased persistence, cell expansion, or tumor trafficking (or a combination of mechanisms) was not determined.
These results are encouraging and suggest a potential way in which anti-tumor T cells might be engineered to make them more active in tumors which express PD-L1/PD-L2. A note of caution in the over interpretation of these results should be taken from the experimental design. The tumor targets used had been engineered to over-express either the target antigen and/or the PD-L1 ligand and the xenograft models do not have the normal repertoire of additional inhibitor cells often found in human tumors (e.g., Treg, MDSC). Furthermore, PD-L1 is often not expressed on tumors, yet is upregulated when an inflammatory response is initiated (e.g., by interferon gamma) and these kinetics of inhibitor response may impact the activity of switch receptor-engineered cells. As also pointed out, tumors tend to have multiple inhibitory factors and it is not known if focusing on one element, such as PD-1/PD-L1, will be sufficient to over a tumor microenvironment that produces inhibitor cytokines (e.g.,TGF-beta) and regulatory cells (e.g., Treg). Overall, the results reported by Liu et al. are encouraging and support continuing research into methods to enhance the activity of anti-tumor engineered T cells as therapy for patients with advanced malignancies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.27). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126-9. [Crossref] [PubMed]
- Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol 2011;29:550-7. [Crossref] [PubMed]
- Cheadle EJ, Sheard V, Hombach AA, et al. Chimeric antigen receptors for T-cell based therapy. Methods Mol Biol 2012;907:645-66. [Crossref] [PubMed]
- June CH, Maus MV, Plesa G, et al. Engineered T cells for cancer therapy. Cancer Immunol Immunother 2014;63:969-75. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38 [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Leen AM, Sukumaran S, Watanabe N, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther 2014;22:1211-20. [PubMed]
- Bollard CM, Rössig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood 2002;99:3179-87. [Crossref] [PubMed]
- Ankri C, Shamalov K, Horovitz-Fried M, et al. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol 2013;191:4121-9. [Crossref] [PubMed]
- Kobold S, Grassmann S, Chaloupka M, et al. Impact of a New Fusion Receptor on PD-1-Mediated Immunosuppression in Adoptive T Cell Therapy. J Natl Cancer Inst 2015;107. [PubMed]
- Prosser ME, Brown CE, Shami AF, et al. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol 2012;51:263-72. [Crossref] [PubMed]
- Moon EK, Wang LC, Dolfi DV, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 2014;20:4262-73. [Crossref] [PubMed]


