
Hypofractionated radiotherapy for breast cancer: too fast or too much?
Background
Hypofractionated schedules are potentially attractive in the treatment of breast carcinoma (1-9). From radiobiological point of view, the linear quadratic model suggests that when the α/β ratio of the tumor is the same or less than that of the critical normal tissue, then a larger dose per fraction (hypofractionation) with a modest decrease in total dose, may be equally or potentially more effective than conventional fractionation. An estimate of 4 Gy for α/β value has been already reported for the fractionation sensitivity of breast cancer (10). The low estimated α/β ratio for breast cancer means that it is probably as sensitive to fraction size as is dose-limiting normal tissue, and hypofractionation for breast cancers may actually be advantageous. Thus, breast cancer seems a promising field for hypofractionated schedules of irradiation.
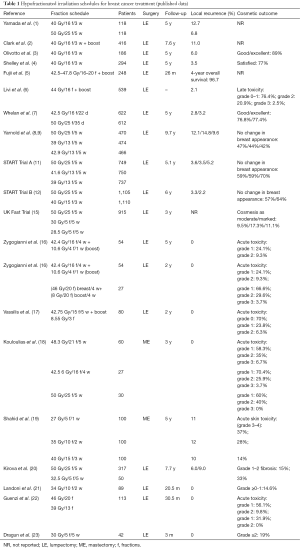
Hypofractionation in breast cancer is the gold standard in UK (11,12). The trials started with Whelan et al. They studied 1,234 patients with T1–T2 N0 disease, while 622 patients received 42.5 Gy in 16 fractions and 612 patients received 50 Gy in 25 factions. Primary endpoint was local recurrence and secondary endpoints were distant recurrence, cosmesis, and late radiation toxicity. With a median follow-up up to 10 years, the local recurrence at 10 years was 6.2% for the hypofractionated schedule and 6.7% for the standard one. Excellent cosmesis at 10 years, according European Organisation for Research and Treatment of Cancer (EORTC) rating system, was 70% with hypofractionation and 71% with standard fractionation (13).
Between 1999 and 2002, 4,451 women with completely excised invasive breast cancer (T1–3, N0–1, M0) were randomized after primary surgery to either 50 Gy in 25 fractions over 5 weeks or 41.6 Gy in 13 fractions or 39 Gy in 13 fractions over 5 weeks (START A). Additionally, patients were also randomized to either 50 Gy in 25 fractions over 5 weeks or 40 Gy in 15 fractions over 3 weeks (START B). Women were eligible if aged over 18 years and did not have an immediate surgical reconstruction. Primary end points were locoregional relapse and late normal tissue effects. Clinician assessments suggested lower 10-year rates of any moderate/marked late normal tissue effects after 39 Gy (43.9%; 95% CI, 39.3–48.7) and similar rates after 41.6 Gy (49.5%; 95% CI, 44.9–54.3) compared with 50 Gy (50.4%; 95% CI, 45.8–55.3) in START A and lower rates after 40 Gy in START B (37.9%; 95% CI, 34.5–41.5) compared with 50 Gy (45.3%; 95% CI, 41.7–49.0) (14).
The START trials showed that hypofractionation was effective. However the question how much the dose per fraction could be increased before adverse acute normal tissue reactions became intolerably high, was investigated by UK FAST trial. The study included 50 Gy in 25 fractions over 5 weeks in comparison with either 30 or 28.5 Gy in 5 fractions over 5 weeks. Acute and late tissue toxicities were evaluated, with the primary end point being adverse effects in the breast. The median follow-up was 3 years and the schedule of 28.5 Gy was similar in toxicity with 50 Gy schedule but lower than the 30 Gy schedule due to the fact that the total dose to the breast was lower (15).
Our experience in the hypofractionation schedules consists in non-randomized trials. We initially applied postoperative radiotherapy after lumpectomy and axillary dissection. From May 2003 to May 2005, 54 patients with stage I–II invasive breast cancer received radiotherapy with 6 MV linear accelerator at a total tumor dose of 53 Gy (equivalent dose-EQD2, 60 Gy), 265 cGy per fraction, in 20 fractions, over 25 days. Acute and late effects as well as cosmetic results assessed using the EORTC and Radiation Therapy Oncology Group. Cosmetic rating system and mammograms performed before radiotherapy, after 6 months and then yearly thereafter. In addition important outcomes including local recurrence, long-term cosmetics and toxicity were assessed. Approximately 98.1% of patients demonstrated a good or excellent cosmetic outcome at 18 months and no difference was detected between treatment groups. In a median follow-up of 5 years no recurrence, local or distance was observed (16). Following, we used two irradiation hypofractionated schedules in the application of tumor bed boost by using two different planning techniques. Eighty-one patients were evaluated between May 2004 and December 2010. The patients were divided for concomitant or sequential boost for tumor bed (Table 1). We applied whole breast irradiation therapy with 3DRT technique, while the tumor boost was delivered with either sequential or concomitant mode. The comparison of the two method showed that the skin toxicity score was worse in the integrated boost group up to 12 months from the end of radiotherapy. No recurrence was observed during the follow-up (16).
Full table
Another trial reported the application of a hypofractionated schedule involved 3 times a week in 80 patients with stage I–II. We delivered 42.75 Gy in 15 fractions over 5 weeks plus an additional boost dose to the tumor bed of 8.55 Gy in 3 fractions using 6 MV photons. We observed late radiation toxicity 1 year after the end of radiotherapy in 2/80 (2.5%) patients. No local recurrence and distal metastasis were noticed (17).
Kirova et al. analyzed 367 elderly women treated by breast conserving surgery and RT for T1–T2 breast cancer, between 1995 and 1999 (20). Finally, 317 patients received 50 Gy in 25 fractions plus boost or not, while 50 patients received 32.5 Gy in 6 fractions of 6.5 Gy delivered once weekly. The median follow-up was 93 months. The evaluation included the long-term cause specific survival (CSS), locoregional recurrence free survival (LRFS), and metastases free survival (MFS) in breast cancer patients. The 5- and 7-year CSS, LRFS and MFS rates were similar in both groups. The acute skin toxicities were acceptable and without differences in the two groups. Late toxicities, grade 1–2 fibrosis, observed in 15% of standard fractionation and 33% of hypofractionated schedule (20).
Landoni et al. showed that late skin toxicity is totally dependent on the dose of different irradiated regions. These results were analyzed by ultrasound examination (21). Guenzi et al. used two different hypofractionated radiotherapy schedules for ductal carcinoma in situ. The first schedule was 46 Gy in 20 fractions of 2.3 Gy 4 times a week and the second one was 39 Gy in 13 fractions of 3 Gy 4 times a week. The results of the study confirmed the superiority of longer schedule; however the toxicity of shorter scheme is acceptable (22).
There are benefits in reducing the number of fractions. Dragun et al. reported a phase II trial of once weekly hypofractionated breast radiotherapy. All patients received 30 Gy in 5 weekly fractions with or without boost. Acute toxicity was well tolerated, although concerns remain about recurrence and overall survival (23).
The German Gynecological Oncology Working Group (AGO) and the German Society for Radiotherapy and Oncology (DEGRO) recently published a consensus guideline for the use of hypofractionated radiotherapy in breast cancer. Based on these guidelines, in patients 40–65 years old could receive hypofractionation scheme with sequential boost and in low risk elderly patients the admission of radiotherapy can be done by the same mode but without boost. If radiotherapy of the regional lymph nodes is included, conventionally fractionated RT (25–28 fractions) must be done (24).
Post-mastectomy hypofractionated irradiation has not been studied as thoroughly as the post conventional surgery radiotherapy. In a retrospective trial by Kouloulias et al., three groups of different schemes (one conventional and two hypofractionated) showed similar results in all arms and enhances the hypothesis that larger fractions are equally effective in controlling the locoregional disease (18). In the study of Shahid et al. (19) of 300 post-mastectomy patients with breast cancer, were randomized to be irradiated with Co60 unit to either 27 Gy in 5 fractions (1 week) arm A, or 35 Gy in 10 fractions (2 weeks) arm B or 40 Gy in 15 fractions (3 weeks) arm C. The locoregional relapses were 11%, 12% and 10% in arms A, B and C, respectively. Moreover, G3 and G4 skin toxicities were 37%, 28% and 14%, respectively.
Too fast or too much?
We read with great interest the article of Brunt et al. (25) concerning the irradiation with different schedules of either 40 Gy/15 fractions/3-week, or 27 Gy/5 fractions/1-week or 26 Gy/5 fractions/1-week. Acute breast skin reactions were graded using either RTOG or CTCAE criteria v4.03. The evaluation of acute toxicity was performed weekly during treatment and for 4 weeks thereafter. Including 190 patients, the first sub-analysis using the RTOG criteria showed for the three schemes 13.6%, 9.8% and 5.8% grade 3 toxicity, respectively. Including 162 patients in the second sub-study, the CTCAE criteria showed 0%, 2.4% and 0% of grade 3 toxicity, respectively.
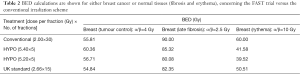
In order to evaluate the potential clinical outcome of the three schedules, we used the radiobiological formulation based on the LQ model. Biologically effective dose for tumor was calculated according to the formula derived from the linear quadratic model including the repopulation effect correction. This correction must be taken into account for post-operative breast tumors treated with radiotherapy because surgical resection can leave behind handful of viable cells which, because are then well vascularized, are capable of rapid growth (17).
Where d is the fraction size (Gy) and n is the number of fractions. T is overall treatment time. Td is delay time to onset of accelerated repopulation. From literature (Wyatt) an effective doubling time Teff of 26 days is assumed to start immediately after surgery and Td is considered as zero. K (Gy/day) is the biological dose per day required to compensate for ongoing tumour cell repopulation, calculated based on Tpot (potential doubling time) and α (radiosensitivity coefficient):
From literature Tpot =14 days and α=0.08 (10,17).
In Table 2, BED calculation are shown for both the three hypofractionation schemes used in publication of Brunt et al. compared with the conventional scheme. The BED calculation for breast tumour shown in the first column includes the appropriate correction for repopulation. For the normal tissues no correction for repopulation was assessed. In general terms, it seems that the weekly schedule consisted of 5.4 Gy per fraction has the highest clinical benefit in terms of effective dose to the cancerous tissue (60.35 Gy), whereas the highest effective dose relevant to fibrosis should be taken into account. The investigators should be aware of this potential clinical benefit by means of that the pending long-term follow-up might show higher rates of late toxicity. The second weekly scheme of 5.2 Gy with 5 fractions seems more safe in terms of either acute (erythema) or late (fibrosis) effect. Surprisingly, the conventional schedule seems to have the highest effective dose in terms of erythema or fibrosis. The investigators showed that the acute toxicity of both weekly schedules was mild, which is in accordance with our radiobiological calculations.
Full table
In any case, as long-term results of the current phase III trials are pending, we still have to conform to current guidelines concerning the prescription of a hypofractionated schedule, as suggested by Budach et al. (24). In conclusion, by radiobiological point of view, although we have to wait for the long-term follow-up, it seems that the scheme with 5.2 Gy per fraction seems even safer than the standard hypofractionated schedule of 2.66 Gy × 15. Long-term follow-up is absolutely mandatory. Do we really talking about the triumph of radiobiology in terms of the LQ model? History will show!
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, the First Affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamada Y, Ackerman I, Franssen E, et al. Does the dose fractionation schedule influence local control of adjuvant radiotherapy for early stage breast cancer? Int J Radiat Oncol Biol Phys 1999;44:99-104. [Crossref] [PubMed]
- Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst 1996;88:1659-64. [Crossref] [PubMed]
- Olivotto IA, Weir LM, Kim-Sing C, et al. Late cosmetic results of short fractionation for breast conservation. Radiother Oncol 1996;41:7-13. [Crossref] [PubMed]
- Shelley W, Brundage M, Hayter C, et al. A shorter fractionation schedule for postlumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys 2000;47:1219-28. [Crossref] [PubMed]
- Fujii O, Hiratsuka J, Nagase N, et al. Whole-breast radiotherapy with shorter fractionation schedules following breast-conserving surgery: short-term morbidity and preliminary outcomes. Breast Cancer 2008;15:86-92. [Crossref] [PubMed]
- Livi L, Stefanacci M, Scoccianti S, et al. Adjuvant hypofractionated radiation therapy for breast cancer after conserving surgery. Clin Oncol (R Coll Radiol) 2007;19:120-4. [Crossref] [PubMed]
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. [Crossref] [PubMed]
- Yarnold J, Bloomeld D, LeVay J. Prospective randomized trial testing 5.7 Gy and 6.0 Gy fractions of whole breast radiotherapy in women with early breast cancer (FAST) trial. Clin Oncol 2004;16:s30.
- Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol 2005;75:9-17. [Crossref] [PubMed]
- Kurtz JM. The clinical radiobiology of breast cancer radiotherapy. Radiother Oncol 2005;75:6-8. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- FAST Trialists group. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93-100. [Crossref] [PubMed]
- Zygogianni A, Kouloulias V, Kyrgias G, et al. Comparison of two radiotherapeutic hypofractionated schedules in the application of tumor bed boost. Clin Breast Cancer 2013;13:292-8. [Crossref] [PubMed]
- Vassilis K, Ioannis G, Anna Z, et al. A unique hypofractionated radiotherapy schedule with 51.3 Gy in 18 fractions three times per week for early breast cancer: outcomes including local control, acute and late skin toxicity. Breast Cancer 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Kouloulias V, Mosa E, Zygogianni A, et al. A Retrospective Analysis of Toxicity and Efficacy for 2 Hypofractionated Irradiation Schedules Versus a Conventional one for Post-Mastectomy Adjuvant Radiotherapy in Breast Cancer. Breast Care 2016; [Epub ahead of print]. [Crossref]
- Shahid A, Athar MA, Asghar S, et al. Post mastectomy adjuvant radiotherapy in breast cancer: a comparision of three hypofractionated protocols. J Pak Med Assoc 2009;59:282-7. [PubMed]
- Kirova YM, Campana F, Savignoni A, et al. Breast-conserving treatment in the elderly: long-term results of adjuvant hypofractionated and normofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2009;75:76-81. [Crossref] [PubMed]
- Landoni V, Giordano C, Marsella A, et al. Evidence from a breast cancer hypofractionated schedule: late skin toxicity assessed by ultrasound. J Exp Clin Cancer Res 2013;32:80. [Crossref] [PubMed]
- Guenzi M, Giannelli F, Bosetti D, et al. Two different hypofractionated breast radiotherapy schedules for 113 patients with ductal carcinoma in situ: preliminary results. Anticancer Res 2013;33:3503-7. [PubMed]
- Dragun AE, Quillo AR, Riley EC, et al. A phase 2 trial of once-weekly hypofractionated breast irradiation: first report of acute toxicity, feasibility, and patient satisfaction. Int J Radiat Oncol Biol Phys 2013;85:e123-8. [Crossref] [PubMed]
- Budach W, Bölke E, Matuschek C. Hypofractionated Radiotherapy as Adjuvant Treatment in Early Breast Cancer. A Review and Meta-Analysis of Randomized Controlled Trials. Breast Care (Basel) 2015;10:240-5. [Crossref] [PubMed]
- Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 2016;120:114-8. [Crossref] [PubMed]


