
Intravenous and intraperitoneal paclitaxel with S-1: new hope for patients with pancreatic cancer and peritoneal metastases?
Pancreatic ductal adenocarcinoma (PDAC) has a dismal prognosis with a 5-year survival rate still below 7% (1) and is predicted to become the second leading cause of cancer-related mortality within the next two decades (2). Surgical resection in combination with systemic therapy offers the only chance of long-term survival or cure (3). However, at diagnosis only 10–20% of patients have resectable tumors, 30–40% have locally advanced borderline-resectable or unresectable tumors, and the majority of 50–60% of patients present with metastatic disease, frequently with peritoneal metastases (1). Patients with peritoneal metastases have an extremely poor prognosis with survival rates ranging from weeks to several months dependent on the extent of the disease, performance status, and therapy. Peritoneal metastases are frequently associated with severe complications such as intestinal obstruction, massive ascites, and malnutrition. These complications result in a poor performance status and hamper the administration of chemotherapy. Moreover, systemically administered chemotherapeutic drugs may not reach sufficient concentrations in the peritoneal cavity and in peritoneal nodules for effective treatment of peritoneal metastases. More effective treatment strategies for peritoneal metastases are, therefore, one of the most pressing needs in the fight against pancreatic cancer.
With this background and encouraged by remarkable results with a combination therapy of intravenous (i.v.) and intraperitoneal (i.p.) paclitaxel (PTX) and S-1 (an oral fluoropyrimidine derivative containing tegafur, gimestat, and otastat potassium) in gastric cancer with peritoneal metastases (4), Satoi et al. conducted a multicenter phase II study to evaluate the same regimen in patients with PDAC and peritoneal metastases. The primary endpoint of this study was 1-year overall survival rate, secondary endpoints were parameters of antitumor effect and safety. Eligibility criteria included histologically proven PDAC, macroscopic peritoneal dissemination or presence of cancer cells on peritoneal cytology in locally unresectable PDAC, chemotherapy-naïve tumors, and patients with ECOG performance status 0 and 1. Exclusion criteria were other distant organ metastases, positive peritoneal cytology without macroscopic dissemination in resectable or borderline-resectable PDAC, other active malignancies, and other severe medical conditions.
After a feasibility study in six patients (unpublished) a total of 33 patients with macroscopic peritoneal metastases (n=22) or locally unresectable PDAC and positive peritoneal cytology (n=11) were included in this study. A peritoneal port access was implanted during surgical exploration or staging laparoscopy. Patients received S-1 orally twice a day at a dosage of 80 mg/m2/d for 14 consecutive days, followed by 7 days without treatment. On day 1 and 8 PTX was administered intravenously at 50 mg/m2 and intraperitoneally at 20 mg/m2 diluted in 1 L of normal saline over 1 hour. This treatment was repeated every 3 weeks for a median of 8.8 months until observation of unacceptable toxicity, disease progression, or conversion surgery. Criteria for conversion surgery were not predefined in the protocol but used upon consensus by the participating surgeons and were a combination of local tumor remission on imaging, decreased tumor markers, disappearance of macroscopic peritoneal metastases upon staging laparoscopy or peritoneal cytology that turned negative.
Given the extremely poor prognosis of patients with PDAC and peritoneal metastases and the oncologic outcome observed with other treatments in the palliative setting (5-11), the outcome reported by Satoi et al. with i.v./i.p. PTX + S-1 is remarkable and very encouraging (Table 1). Satoi et al. observed a median survival time of 16.3 months, and 1- and 2-year survival rates of 62% and 23%, respectively. As further evidence of treatment efficacy, the objective response rate by RECIST criteria was 36%, the disease control rate was 82%, positive peritoneal cytology turned negative in 55%, malignant ascites disappeared in 60%, and CA 19-9 levels decreased in 51% and returned to normal levels in 35% of patients. Notably, 8 (24%) patients (5 with macroscopic peritoneal dissemination and 3 with unresectable tumors and positive peritoneal cytology) met the criteria for conversion surgery and underwent surgical resection. With extended resections including major artery and/or portal vein resections in 5 of 8 cases, an R0 status was achieved in 6 patients and an R1 resection in two patients. The overall median survival in patients with conversion surgery was 27.8 months and significantly longer than in patients who did not undergo resection (14.2 months). The toxicity of the regimen was acceptable. One patient died of a superior mesenteric artery thrombosis after the first treatment infusion, and this was regarded as treatment-related mortality.
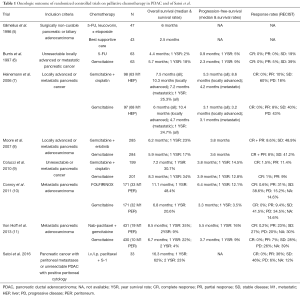
Full table
Satoi et al. have to be commended for their nicely conducted and important study that holds high promise and may represent a milestone in the treatment of PDAC with peritoneal metastasis. However, as the authors discuss, even with its highly promising results, this study can only be hypothesis-generating due to its relatively small size and non-randomized design. Larger observational studies and intelligently designed randomized controlled trials are now warranted to answer open questions, to confirm the promising efficacy of i.v./i.p. PTX + S-1, and to compare this regimen with other treatments.
Several pieces of information that would have been of interest are not reported by Satoi et al.: did they observe differences in survival and efficacy between the subgroups of patients with macroscopic dissemination and positive cytology? While a large retrospective study in 462 patients treated between 1995 and 2005 showed no survival differences between patients who underwent resection with positive peritoneal cytology and of patients who had stage IV disease and were not resected (12), the impact of positive cytology versus macroscopic dissemination on survival may have changed with advances in systemic treatment. In the 22 patients with macroscopic peritoneal metastases the extent of dissemination using one of the available scoring systems for peritoneal surface cancers would have been of interest. Positive cytology versus macroscopic metastases, the extent of macroscopic dissemination, and other prognostic factors such as CA 19-9 levels will be important parameters to be used in future studies for patient stratification and to identify patient subgroups that benefit most from i.p. chemotherapy. It would also have been of interest to report the progression pattern (peritoneal or other organs) and the cause of death in the 23 patients who had died during the study. Finally, given the remarkable survival observed especially after conversion surgery, information on further cancer-directed therapy and disease status in this patient subgroup is of interest. Such information would certainly further stimulate and facilitate the design of future studies that are now necessary.
Based on randomized controlled trials the combination regimen FOLFIRINOX has been identified as highly effective in metastatic PDAC (10). Recently, several large observational studies and a patient-level meta-analysis found that FOLFIRINOX is also highly effective in locally advanced, unresectable PDAC (13-15). Of note, one of these studies also included 47.2% (59/125) patients who reached criteria for conversion surgery after FOLFIRINOX treatment for metastatic disease, 76 (60.8% of patients with FOLFIRINOX) underwent resection and the median survival was 16.0 months after resection (22.6 months after initiation of therapy) (14). Based on these data a randomized controlled trial of i.v./i.p. PTX + S-1 versus systemic FOLFIRINOX for PDAC with peritoneal metastases (and ECOG 0 and 1 status) may be one possible path forward.
But can the remarkable results observed with S-1 in Asian PDAC patients in randomized controlled trials in the palliative and adjuvant settings (16,17), and now in the study by Satoi et al., be extrapolated to patients of other ethnicities? The activity of cytochrome P-450 2A6, which is the key enzyme in converting Tegafur to 5-FU (18) is different between Japanese and Caucasians (19) and this fact may contribute to a higher gastrointestinal toxicity and lower tolerable doses of S1 in Caucasians (20). While S1 is commonly used in Asia, studies on its tolerability and efficacy in non-Asian populations are currently lacking. Therefore, the regimen used by Satoi et al. may not be applicable and may need to be modified in non-Asian patient populations.
Interdisciplinary treatment including surgical resection remains the only hope of long-term survival for patients with PDAC. With progress in both surgery and systemic therapy (3) the indications for surgical resection in high-volume centers have been extended towards extended resections in locally advanced PDAC (21,22) resection after neoadjuvant treatment for primarily unresectable PDAC (13-15,23), and re-resection for isolated local recurrence of PDAC (24). In highly selected patients even resections of hepatic oligometastatic PDAC are performed (25). With a rate of conversion surgery and resection of 24% in patients which initially presented with peritoneal metastases the study by Satoi et al. extends the possible indications for surgical resection even further. It is justified and necessary to test the concept of conversion surgery in patients exhibiting good treatment responses with remission to a resectable stage. However, the remarkable results observed by Satoi et al. after conversion surgical resection have to be interpreted with caution, because they may to a large extent be explained by selection of patients with favorable prognostic parameters for surgery. While the true impact of conversion surgery remains to be shown, Satoi et al. clearly demonstrate a promising efficacy of i.p. chemotherapy with respect to both overall survival and control of peritoneal metastases. This is highly relevant for effective palliation of patients with peritoneal metastases and may have a considerable impact on their quality of live and quality-adjusted survival, parameters that should be assessed in future studies.
In conclusion Satoi et al. have shown encouraging clinical efficacy of i.v./i.p. PTX + S1 in Asian patients with PDAC and peritoneal metastases. This study provides new hope for patients with PDAC and peritoneal dissemination and will hopefully trigger further studies to improve the treatment for these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiaoping Yi (Department of Radiology, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Hartwig W, Werner J, Jäger D, et al. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013;14:e476-85. [Crossref] [PubMed]
- Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010;21:67-70. [Crossref] [PubMed]
- Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946-52. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 2010;28:1645-51. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg 2006;10:1347-53. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg 2016;264:457-63. [Crossref] [PubMed]
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-10. [Crossref] [PubMed]
- Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Ikeda K, Yoshisue K, Matsushima E, et al. Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res 2000;6:4409-15. [PubMed]
- Shimada T, Yamazaki H, Guengerich FP. Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica 1996;26:395-403. [Crossref] [PubMed]
- Chuah B, Goh BC, Lee SC, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 2011;102:478-83. [Crossref] [PubMed]
- Hartwig W, Hackert T, Hinz U, et al. Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg 2009;250:81-7. [Crossref] [PubMed]
- Hartwig W, Vollmer CM, Fingerhut A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 2014;156:1-14. [Crossref] [PubMed]
- Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, "unresectable" pancreatic cancer. Surgery 2012;152:S33-42. [Crossref] [PubMed]
- Strobel O, Hartwig W, Hackert T, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol 2013;20:964-72. [Crossref] [PubMed]
- Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016;160:136-44. [Crossref] [PubMed]


