
Is inhibiting the DNA damage response the answer to treatment resistance in glioma stem cells?
Treatment options for GBM patients
Treatment outcomes for patients newly diagnosed with aggressive stage IV glioma (GBM) have not improved since Stupp and colleagues published the 5-year follow up results of their GBM trial (n=573) in 2009. This trial compared the effect on survival of adding the alkylating drug, temozolomide, daily during radiation therapy followed by at least 6 cycles of temozolomide (5 days per 28 days) to debulking surgery plus conventional radiation therapy. Adding temozolomide increased the mean overall survival from 11 to 15 months and patient survival at 2 years from 11% to 27% (1). Other clinicians using the Stupp protocol have reported a mean overall survival of 20–21 months (2,3). The addition of targeted drugs, which had shown great promise in preclinical studies, has failed to increase the overall survival beyond 23 months after diagnosis (4-6). Eventually, all patients relapse and undergo salvage therapy. The most promising salvage options currently are radiosurgery (7) or hypo-fractionated radiation therapy (8) which can add an extra 12 months to patients’ lives. Treatment for GBM is palliative and not curative, therefore patient quality of life is as important as the extra few months of life for patients and their families. Tumor and treatment related side effects are diverse and can be debilitating depending on tumor location and treatment choice. The acute effects of radiation, such as swelling of the brain, can be alleviated by the steroidal anti-inflammatory drug, dexamethasone, which has its own toxicity profile. Chronic radiation-induced neurological and cognitive deficits are less of a problem in patients who are not likely to survive long enough to experience them. New treatment modalities for GBM should be aimed at increasing life expectancy by several years, which means that maintaining neurological and cognitive function will be increasingly important. Cancer-specific radiosensitization is the most promising avenue for novel GBM strategies because it promises increased survival along with the possibility of reducing dose to the brain and thus decreasing radiation-induced toxicities. Ionizing radiation kills cells either directly by ionizing parts of the DNA double helix or indirectly by ionizing water molecules, creating highly aggressive hydroxyl radicals in close vicinity to DNA. Hydroxyl radicals can cause base damage, polynucleotide cross linking, single stranded and double stranded breaks. Double stranded breaks are difficult and time consuming to repair and thus contribute most to cell death (9). In response to radiation, cells activate the DNA damage response (DDR), which initiates a series of cascades involving cell cycle checkpoint activation, various forms of DNA repair and, if unsuccessful, inducing apoptosis. GBMs are highly resistant to treatment for a number of reasons that will be discussed in more detail below. The presence of cancer stem cells, the upregulation of growth factor receptor signaling pathways, constitutive activation of the DDR and the ability to switch metabolic phenotypes in response to treatment all contribute to treatment resistance and thus poor prognosis.
Cancer stem cells
The paradigm of tumor progression being driven by the emergence of treatment resistant clones from a more or less static phenotypic background through mutational and epigenetic changes has shifted in the last 20 years. The new concept is that tumors are disorganised loosely hierarchical tissues, composed of many different phenotypes. Tumor progression is driven by a small population of cancer stem cells, who are quiescent, slowly self-renew and able to produce the entire range of phenotypes that makes up the tumor. Cancer stem cells (CSCs) were first described almost two decades ago for acute leukemia (10). Since then, CSCs have been reported in many different types of cancer, including GBM, although their contribution to tumorigenesis is somewhat controversial (4,11). Tumors are likely to contain CSCs with different tumorigenic potential and with a high level of individual plasticity (12). CSCs are more resistant to anti-cancer treatments than proliferating or differentiated cells. In addition, treatment will select the most aggressive treatment-resistant CSC phenotype and/or increase the tumorigenic potential of individual CSCs. Stem cell-like cells in GBMs (GSCs) are particularly resistant to temozolomide (13), radiation (14) and combined treatment (15). Any novel strategy aiming to cure GBM therefore needs to specifically kill GSCs. Measuring stem-cell-ness by the expression of GBM specific stem cell markers is useful to delineate between GSCs and non-GSCs (16-18). Measuring treatment resistance of both GSCs and non-GSCs in paired cell lines originating from the same patient-derived tumor is the most clinically relevant way to test novel treatment strategies.
GBM survival pathways
Even without therapy-induced genotoxic stress, GBMs have very active survival pathways, including the EGFR (epidermal growth factor receptor) and VEGFR (vascular endothelial growth factor receptor) pathways (depicted in Figure 1). Radiation increases the activity of these pathways even more (6,19). The vast majority (80%) of GBMs are driven by mutations in the EGFR/PI3K (phosphatidylinositol-3-kinase)/AKT (protein kinase B)/mTOR (mammalian target of rapamycin) pathway. Most common mutations are in EGFR (40%) and PTEN (phosphatase and tensin homolog) (37%) (20). The effect of growth pathway inhibitors depends to a large extent on the mutational profile of the tumor. GBMs can be divided into four subtypes based on the Cancer Genome Atlas project (4). The classical subtype is highly proliferative with mainly EGFR amplifications/variants and PTEN deletions. The mesenchymal subtype displays mutations in NF-1 (neurofibromatosis type 1) and NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells). The neural subtype is also highly proliferative, whereas the proneural subtype is less proliferative with a better prognosis and mutations in p53, PI3K and PDGFR (platelet derived growth factor receptor A) and IDH1 (isocitrate dehydrogenase 1) (4). The vast majority of primary GBMs belong to the classical subtype. However, GBMs are likely derived from multiple stem cell lineages with different mutational profiles. In addition, treatment affects mutation profiles, selecting for more aggressive phenotypes. In support of this, radiation was shown to shift newly diagnosed proneural phenotypes to a more aggressive mesenchymal phenotype in recurrent GBMs (21). An analysis of treatment-naïve primary tumors and tumors that occurred after treatment with radiation and temozolomide of 38 GBM patients demonstrated that the mutational profile of locally recurring tumors was 70% similar to that of the primary tumors. In contrast, distant recurring tumors only retained about 25% of the mutational profile of the primary tumor (22). These findings are significant as the treatment of recurrent GBMs is generally based on the mutational profile of the primary tumor. Dissection and re-profiling of recurrent tumors will highlight any major changes to the mutation profile and identify therapies most likely to affect the new lesions.
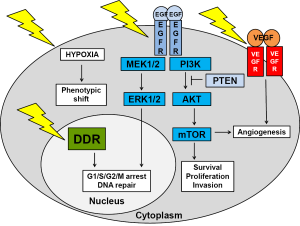
Inhibiting EGFR signaling
Although preclinical studies have shown great promise for a number of inhibitors of the growth factor signaling pathways in preclinical orthotopic GBM rodent models, their effect on patient survival has been disappointing (4-6,19). Because GBMs have the plasticity to change their phenotype, they are likely to increase signaling through one of their other survival pathways. The concept of synthetic lethality involves using a drug that is lethal only against a certain genetic background. An example of synthetic lethality is the increased susceptibility of tumors with intact PTEN to EGFR inhibition. Over-expression of EGFR in 40% of GBMs favors PI3K/AKT signaling (23). EGFRvIII is the most common EGFR variant (20%) and lacks the extracellular domain resulting in a constitutively activated survival pathway (23-25). Gefitinib and erlotinib are both tyrosine kinase inhibitors that bind to intracellular EGFR domain. Tumor responses to these inhibitors were only seen in patients whose tumors had intact PTEN, whilst having no effect on patients whose tumors had lost PTEN (11). Highly active EGFR signaling GBMs produce more reactive oxygen species, causing more DNA damage which is dealt to by increased DNA repair (4). A direct link between the DDR and EGFR signaling networks was shown by Golding and colleagues who reported an inhibition of DSB repair by AKT and ERK (extracellular signal regulated kinase) inhibitors and an increase in DSB repair through EGFR and EGFRvIII signaling in malignant gliomas (23).
Inhibiting angiogenesis
Rather than inhibiting EGFR signaling with its overlapping and possibly redundant branches, targeting tumor blood supply might meet with more success. GBMs are highly vascularized and produce high levels of VEGF (vascular endothelial growth factor) which binds to VEGFR on the membrane of GBM cells and endothelial cells, promoting angiogenesis. However, blood supply to the tumor is compromised as blood vessels are tortuous and leaky with microvascular hyperplasia, leading to transient areas of hypoxia. Anti-angiogenic therapy involves either trapping VEGF (bevacizumab) or blocking VEGFR (sunitinib). In theory, this should decrease the blood supply to the tumor even further, resulting in tumor regression or at least inhibit further tumor growth. However, the increased hypoxia causes a phenotypic shift in energy metabolism from mitochondrial respiration to glycolysis via hypoxia-mediated stabilization of HIF-1α (hypoxia inducible factor 1α). So instead of the expected inhibition of tumor progression, tumor cells became a lot more invasive as was demonstrated in a patient-derived rat GBM model (26). Tumor cells went through an endothelial-mesenchymal transformation which was associated with increased invasion into normal brain tissue and an increase in stem cell marker expression (27). The ability to switch from a highly proliferative poorly invasive phenotype to a low proliferative highly invasive phenotype depending on local oxygen levels contributes to the extreme radiation resistance of GSCs (4,28). Radiation therapy, delivered in sub-lethal fractions, is likely to promote phenotype switching by increasing hypoxia and by activating multiple survival pathways. In light of these preclinical finding, it is not surprising that a recent meta-analysis of seven stage II and three stage III clinical trials reported that combining bevacizumab with the Stupp protocol did not increase patient survival over and above 23 months (29). However, adding bevacizumab improved patient quality of life in some patients because of its corticosteroid-sparing effect (29). A recent Cochrane analysis of seven RCTs (n=2,987) also did not recommend adding bevacizumab to the Stupp regimen in newly diagnosed GBM patients (30). Furthermore, adding the topoisomerase inhibitor, irinotecan and bevacizumab to the Stupp protocol did not increase patient survival in two II RCTs and one stage II single arm study either (31-33).
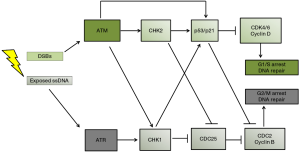
Inhibiting the DNA damage response (DDR)
The previous sections have shown a disappointing lack of clinical response to EGFR and VEGFR inhibitors. Recent attention has therefore shifted to inhibiting another GBM survival pathway: the DDR which is controlled by the activity of two protein kinases, ATM (ataxia telangiectasia mutated) and ATR (ataxia telangiectasia and Rad3-related) (depicted in Figure 2). The DDR is upregulated in high grade gliomas as evidenced by high levels of p53, pATM, pCHK1 (cell cycle checkpoint kinase1), pCHK2 (cell cycle checkpoint kinase 2) and PARP-1 [Poly (ADP-ribose) polymerase 1] (17,35,36). PARP-1 plays an important role in base excision repair, nucleotide excision repair, single and double stranded break repair (37). Any component of the DDR could be considered a good target for multimodal strategies against GBM, a strategy often referred to as cell cycle checkpoint abrogation. Indeed, the number of studies exploring the effect of small DDR inhibitors in combination with radiation and DNA damaging agents in preclinical cancer models has grown substantially in recent years. Adding additional genotoxic stress to the genetically unstable GBMs through radiation or chemotherapy makes p53 mutant tumor cells particularly sensitive to DDR inhibition. With normal surrounding tissue having functional p53, these radio- and chemo-sensitizing effects are not as strong, resulting in a cancer-specific sensitization effect. The effects of specific DDR inhibitors was recently reviewed by Benada and colleagues (34). This perspective summarizes the radiosensitizing effects of specific ATM inhibitors for GBMs. Several authors have reported the effects on GSCs of DDR inhibitors against ATM, ATR, CHK1, CHK2 and PARP-1 (17,20,35,36,38-41). The radiosensitizing effects of first generation (KU 55933) and the more potent second generation (KU60019) ATM inhibitors have been demonstrated in GBM cell lines (39,40) and GSC cell lines in vitro (17,41). Raso and colleagues showed potent radiosensitization by KU-55933 and KU-60019 of GSCs but not non-GSCs using patient-derived paired GSCs and non-GSCs (17). However, as their cell lines had limited clonogenic potential (<5% before treatment) they used MTT assays 8 days after radiation exposure, which measures cellular NADH flux rather than clonogenic potential, which is the only clinically relevant way to determine the radiosensitivity of cell lines (42). Golding and colleagues used clonogenic assays to show that continuous exposure to nanomolar concentrations of KU-60019 radiosensitized established U1242, U87 and U373 GBM cell lines and did not interfere with temozolomide treatment. Carruthers and colleagues also described how DDR inhibition by KU-55933 strongly radiosensitized GSCs in vitro in their paper published in Molecular Oncology 2 years ago. They demonstrated that (I) GSCs are more radioresistant than non-GSCs; (II) that radiation strongly activates ATM induced cell cycle checkpoint activation and DSB repair in GSCs; (III) that KU-55933 reverses radiation-induced DDR activation and DSB repair in GSCs and (IV) that KU-55933 radiosensitizes GSCs to a greater extent than non-GSCs (38). The results of this in vitro study do have clinical relevance as the authors used patient-derived paired GSC and non-GSC cell lines cultured in serum free stem cell medium and stem cell depleting medium, rather than established cell lines. They also used clonogenic assays to measure radiosensitivity rather than viability or metabolic assays. Interestingly, KU-55933 only partially stopped G2/M check point activation in GSCs, which suggests DDR activation through ATR. Further research by this group showed that ATM inhibition caused a more pronounced radiosensitization in GSCs than inhibition of ATR, CHK1 or PARP-1 as single agents. However, inhibiting both PARP-1 and ATR had a stronger radiosensitizing effect than ATM inhibition alone, showing that inhibiting multiple pathways of the DDR is more effective than inhibiting a single pathway (35). Biddlestone-Thorpe and colleagues were the first group to demonstrate potent radiosensitization of KU-60019 in vivo. They used U1242/luc-GFP cells that form highly invasive and highly mitotic tumors in brains of athymic mice. Nanomolar concentrations of KU-60019 were delivered using convection-enhanced delivery infusion, immediately followed by 3 Gy radiation on 3 separate days. They found that mice with mutated p53 gliomas lived more than 160 days longer after combined treatment compared with radiation alone. Mice with wild type p53 gliomas only lived 16 days longer when receiving combined treatment compared with radiation alone (40). This is another example of synthetic lethality with ATM inhibition being much more effective in tumors that lack functional p53 or PARP-1 (35,40). With respect to the effects of ATM inhibitors on normal brain tissue, Golding and colleagues co-cultured U1242 glioma cells with astrocytes in the absence of radiation. They reported that KU-60019 and temozolomide alone or in combination had no effect on astrocytes but reduced glioma cell growth by 40–50% after either KU-60019 or temozolomide and by 70% after combined treatment (39). Vecchio and colleagues addressed the issue of safety by injecting different concentrations of KU-60019 into the brain of healthy mice immediately followed by a single dose of 2.5 Gy radiation. Neither this short-term exposure not prolonged exposure using osmotic mini-pumps and re-absorbable clots caused neurological symptoms, macroscopic changes or histopathological alterations in brain, bone marrow, heart, kidney, liver, lungs, spleen and testes in healthy mice. Although the safety aspects of ATM inhibitors need to be assessed more thoroughly the future, the lack of damage to multiple organs holds great promise for future clinical studies (41).
Concluding remarks
A better understanding of the complexity of GBM tumors, as diverse disorganized tissues consisting of many phenotypes, including GSCs, has led to an appreciation of the plasticity of GBMs. Treatment resistance is further characterized by the ability to upregulate different survival pathways, including EGFR and VEGFR signaling and the DDR and switch metabolic phenotypes when necessary (summarized in Figure 3). After the failure of many EGFR and VEGFR pathways inhibitors to increase patient survival, recent research efforts have focused on inhibiting components of the DDR to sensitize various cancer types to DNA damaging therapies by interfering with cell cycle checkpoint activation. Potent radiosensitization of ATM inhibitors on GSCs in vitro and in orthotopic mouse GBM models, together with a good safety profile to date, makes them prime candidates for progression into clinical trials. Multimodal strategies, including radiation, temozolomide and DDR inhibition for p53null/PTENwt GBMs/GSCs, may deliver a strong enough clinical response to allow a decrease of radiation dose to the brain, maintaining better cognitive and neurological function, whilst substantially increasing survival of GBM patients.

Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.71). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [Crossref] [PubMed]
- Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2011;29:142-8. [Crossref] [PubMed]
- Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 2009;27:3861-7. [Crossref] [PubMed]
- Bartek J Jr, Ng K, Bartek J, et al. Key concepts in glioblastoma therapy. J Neurol Neurosurg Psychiatry 2012;83:753-60. [Crossref] [PubMed]
- Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med 2014;6:1359-70. [Crossref] [PubMed]
- Scaringi C, Enrici RM, Minniti G. Combining molecular targeted agents with radiation therapy for malignant gliomas. Onco Targets Ther 2013;6:1079-95. [PubMed]
- Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2012;82:2018-24. [Crossref] [PubMed]
- Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol 2013;111:187-94. [Crossref] [PubMed]
- Wouters B, Begg A. Irradiation-induced damage and the DNA damage response. In: Joiner MC, van der Kogel AJ, editors. Basic Clinical Radiobiology. Fourth. London: Hodder & Arnold 2009:11–41.
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [Crossref] [PubMed]
- Knights MJ, Kyle S, Ismail A. Characteristic features of stem cells in glioblastomas: from cellular biology to genetics. Brain Pathol 2012;22:592-606. [Crossref] [PubMed]
- Herst PM, Berridge MV. Cell hierarchy, metabolic flexibility and systems approaches to cancer treatment. Curr Pharm Biotechnol 2013;14:289-99. [Crossref] [PubMed]
- Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012;488:522-6. [Crossref] [PubMed]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756-60. [Crossref] [PubMed]
- Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol 2008;26:3015-24. [Crossref] [PubMed]
- Broadley KW, Hunn MK, Farrand KJ, et al. Side population is not necessary or sufficient for a cancer stem cell phenotype in glioblastoma multiforme. Stem Cells 2011;29:452-61. [Crossref] [PubMed]
- Raso A, Vecchio D, Cappelli E, et al. Characterization of glioma stem cells through multiple stem cell markers and their specific sensitization to double-strand break-inducing agents by pharmacological inhibition of ataxia telangiectasia mutated protein. Brain Pathol 2012;22:677-88. [Crossref] [PubMed]
- Ropolo M, Daga A, Griffero F, et al. Comparative analysis of DNA repair in stem and nonstem glioma cell cultures. Mol Cancer Res 2009;7:383-92. [Crossref] [PubMed]
- Hein AL, Ouellette MM, Yan Y. Radiation-induced signaling pathways that promote cancer cell survival Int J Oncol 2014;45:1813-9. (review). [PubMed]
- Signore M, Pelacchi F, di Martino S, et al. Combined PDK1 and CHK1 inhibition is required to kill glioblastoma stem-like cells in vitro and in vivo. Cell Death Dis 2014;5:e1223 [Crossref] [PubMed]
- Bartkova J, Hamerlik P, Stockhausen MT, et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene 2010;29:5095-102. [Crossref] [PubMed]
- Kim J, Lee IH, Cho HJ, et al. Spatiotemporal Evolution of the Primary Glioblastoma Genome. Cancer Cell 2015;28:318-28. [Crossref] [PubMed]
- Golding SE, Morgan RN, Adams BR, et al. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther 2009;8:730-8. [Crossref] [PubMed]
- Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci 2009;16:748-54. [Crossref] [PubMed]
- Mukherjee B, McEllin B, Camacho CV, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res 2009;69:4252-9. [Crossref] [PubMed]
- Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A 2011;108:3749-54. [Crossref] [PubMed]
- Piao Y, Liang J, Holmes L, et al. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro Oncol 2012;14:1379-92. [Crossref] [PubMed]
- Ichikawa T, Otani Y, Kurozumi K, et al. Phenotypic Transition as a Survival Strategy of Glioma. Neurol Med Chir (Tokyo) 2016;56:387-95. [Crossref] [PubMed]
- Poulsen HS, Urup T, Michaelsen SR, et al. The impact of bevacizumab treatment on survival and quality of life in newly diagnosed glioblastoma patients. Cancer Manag Res 2014;6:373-87. [Crossref] [PubMed]
- Khasraw M, Ameratunga MS, Grant R, et al. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev 2014;CD008218 [PubMed]
- Hofland KF, Hansen S, Sorensen M, et al. Neoadjuvant bevacizumab and irinotecan versus bevacizumab and temozolomide followed by concomitant chemoradiotherapy in newly diagnosed glioblastoma multiforme: A randomized phase II study. Acta Oncol 2014;53:939-44. [Crossref] [PubMed]
- Chauffert B, Feuvret L, Bonnetain F, et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: final results of the TEMAVIR study from ANOCEF†. Ann Oncol 2014;25:1442-7. [Crossref] [PubMed]
- Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res 2011;17:4119-24. [Crossref] [PubMed]
- Benada J, Macurek L. Targeting the Checkpoint to Kill Cancer Cells. Biomolecules 2015;5:1912-37. [Crossref] [PubMed]
- Ahmed SU, Carruthers R, Gilmour L, et al. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res 2015;75:4416-28. [Crossref] [PubMed]
- Majuelos-Melguizo J, Rodríguez MI, López-Jiménez L, et al. PARP targeting counteracts gliomagenesis through induction of mitotic catastrophe and aggravation of deficiency in homologous recombination in PTEN-mutant glioma. Oncotarget 2015;6:4790-803. [Crossref] [PubMed]
- Jubin T, Kadam A, Jariwala M, et al. The PARP family: insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival. Cell Prolif 2016;49:421-37. [Crossref] [PubMed]
- Carruthers R, Ahmed SU, Strathdee K, et al. Abrogation of radioresistance in glioblastoma stem-like cells by inhibition of ATM kinase. Mol Oncol 2015;9:192-203. [Crossref] [PubMed]
- Golding SE, Rosenberg E, Adams BR, et al. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle 2012;11:1167-73. [Crossref] [PubMed]
- Biddlestone-Thorpe L, Sajjad M, Rosenberg E, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res 2013;19:3189-200. [Crossref] [PubMed]
- Vecchio D, Daga A, Carra E, et al. Predictability, efficacy and safety of radiosensitization of glioblastoma-initiating cells by the ATM inhibitor KU-60019. Int J Cancer 2014;135:479-91. [Crossref] [PubMed]
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 2005;11:127-52. [Crossref] [PubMed]


